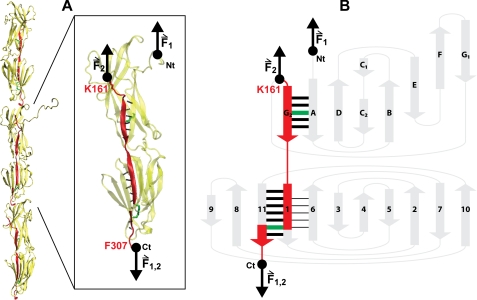
FIGURE 4.
Mechanical architecture of Spy0128. A, model of the pili in S. pyogenes showing the spatial arrangement of three Spy0128 units (21). The structure of one Spy0128 monomer (PDB code: 3B2M) is enlarged. The hydrogen bonds involved in the mechanical stabilization of the domains are shown as dotted black lines. The intramolecular isopeptide bonds (Lys-36—Asn-168; Lys-179—Asn-303) are depicted in green. Pilin monomers homopolymerize to produce the pilus shaft through intermolecular isopeptide bonds between Lys-161 and the carboxyl group of Thr-311 (21). As a consequence of both the inter- and intramolecular isopeptide bonds, an axial force applied to the pilus is transmitted along the chain colored in red (instead of Thr-311, the last residue modeled in the crystal structure of Spy0128, Phe-307, is shown). The pulling directions in the polyproteins used in this work (F1) and in native pili (F2) are indicated by black arrows. VMD was used to prepare the figure (49). B, topology diagram of Spy0128. The main mechanical transitions states are represented by thick black bars. The mechanical transition state giving rise to the intermediate captured in the mechanical unfolding of Spy0128 E258A is represented by black lines between strands 1 and 6. Force pathways and intramolecular isopeptide bonds are colored as in A. The N and C termini are indicated in both panels.