FIGURE 3.
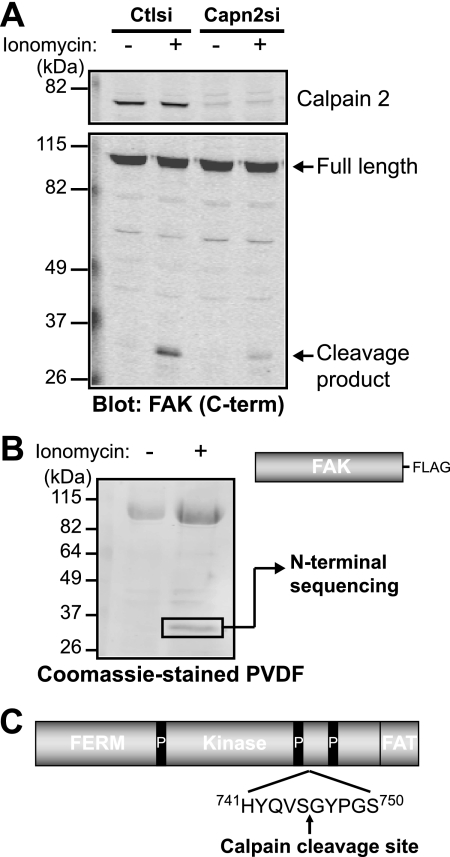
Calpain cleaves FAK between the two C-terminal proline-rich regions. A, HEK 293 control (Ctlsi) or calpain 2 (Capn2si) siRNA cells were treated with vehicle (−) or 1 μm ionomycin + 1 mm CaCl2 (+). Cell lysates were analyzed by immunoblotting and probed for calpain 2 and FAK. Immunoblots shown are from one of two independent experiments. C-term, C-terminal. B, FAK-FLAG was transiently transfected into HEK 293 cells, which were treated with vehicle (−) or 1 μm ionomycin + 1 mm CaCl2 (+). Cell lysates were incubated with anti-FLAG beads, and protein complexes were transferred to polyvinylidene difluoride membrane. The 35-kDa band was analyzed by N-terminal sequencing. The Coomassie Blue-stained polyvinylidene difluoride (PVDF) membrane shown is from one of three independent experiments. C, a schematic of FAK domain organization (4.1, ezrin, radixin, moesin (FERM); kinase; focal adhesion targeting (FAT)) denotes the site of calpain cleavage after Ser-745, between the two C-terminal proline-rich (P) regions.