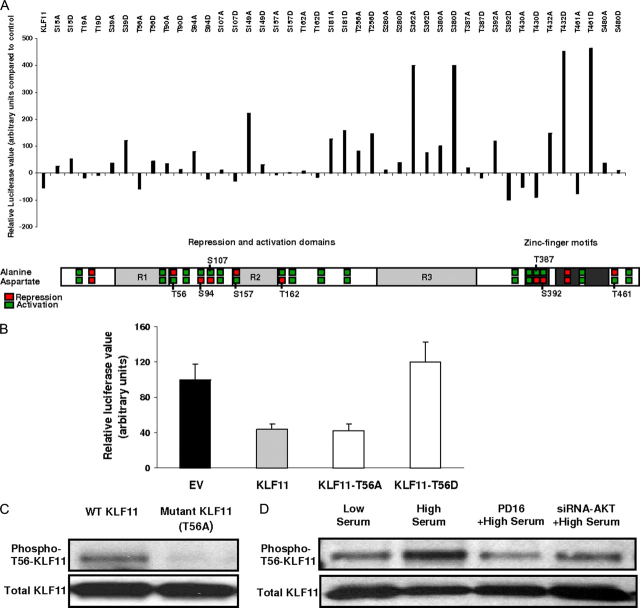
FIGURE 5.
Post-translational modification of threonine at position 56 in KLF11, a target of phosphorylation by AKT, is crucial in KLF11-mediated repression of the cPLA2α promoter.A, Chinese hamster ovary cells were co-transfected with the cPLA2α promoter reporter construct along with either EV or KLF11 constructs from a library of mutant KLF11 proteins where serines and threonines were replaced with either alanines or aspartic acids as indicated. The eight phosphomimetic and non-phosphorylatable KLF11 mutants with opposing effects on cPLA2α promoter activity are displayed along with a KLF11 protein domain outline. B, FLO cells co-transfected with the cPLA2α promoter reporter construct along with either empty vector or a phosphomimetic (T56D) or non-phosphorylatable (T56A) KLF11 mutant in the R1 domain (in close proximity of its Sin3a binding site) shows that at 48 h, compared with control, wild-type KLF11 repressed the cPLA2α promoter activity to 43 ± 6% but the phosphomimetic T56D-KLF11 mutant resulted in a complete release of cPLA2α promoter repression by KLF11 (120 ± 23%). The repression of cPLA2α persisted with the T56A-KLF11 mutant (42 ± 7%). C, lysates from Chinese hamster ovary cells transfected with wild-type KLF11 or T56A mutant KLF11 after immunoprecipitation of His-tagged KLF11 followed by Western blot with phospho-Thr-56-KLF11 antibody shows the specificity of this antibody as it does not bind to non-phosphorylatable T56A-KLF11. D, KLF11-transfected FLO cells were treated with either scrambled siRNA + vehicle or siRNA against AKT (AKT-1, -2, and -3) transfection or PD168393 (EGFR blocker) to inhibit AKT. 24 h later cells were either maintained in 5% FBS (low serum) or given a 90-min pulse of high serum medium (10% FBS to activate EGFR-AKT pathway). After immunoprecipitating His-tagged KLF11 protein, resolving by 10% SDS-PAGE, and immunoblotting with anti-phospho-Thr-56-KLF11 and total KLF11, as a loading control, we found that the high serum pulse that activates AKT (data shown in supplemental Fig. S6, 2 and 3) results in phosphorylation of Thr-56 in KLF11 and that the siRNA against AKT, as well as PD168393 to inhibit AKT, markedly reduced the phosphorylation of this site.