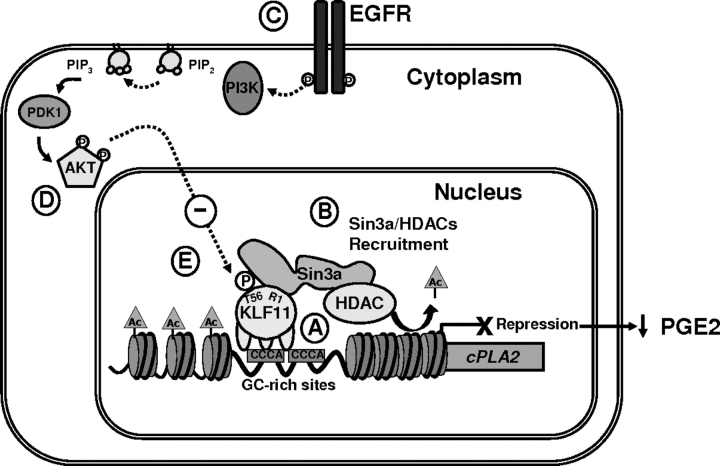
FIGURE 7.
Mechanistic model of KLF11-mediated tumor suppression and its antagonism by an oncogenic pathway. KLF11 binds to the GC-rich consensus sequences in the promoter region of cPLA2α, the key rate-limiting enzyme of the oncogenic PGE2 cascade. A, KLF11 represses the cPLA2α promoter by recruiting the chromatin remodeling complex, Sin3a-HDAC (B). As a consequence, KLF11 behaves as a tumor suppressor in Barrett's epithelial cells, at least in part, by repression of the cPLA2α-PGE2 pathway. EGFR-AKT signaling, which is up-regulated in a subset of patients during carcinogenesis in Barrett's esophagus (C) phosphorylates threonine at position 56 in the R1 domain of KLF11 in the immediate vicinity of its Sin3a interacting domain (D), and reverses the KLF11-dependent repression of the cPLA2α promoter (E). Together, this model outlines mechanistic links, namely KLF11-SIN3a/HDAC-cPLA2α-PGE2 and EGFR/AKT-KLF11 (Thr-56 phosphorylation)-SIN3a/HDAC-cPLA2α-PGE2.