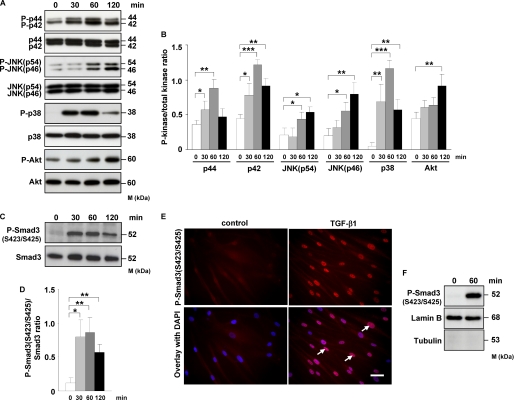
FIGURE 2.
TGF-β1 induces phosphorylation of MAPK, Akt, and Smad3, as well as translocation of phospho-Smad3 into the nucleus of HLF. A, HLF were treated for the indicated time points with TGF-β1, and the activation of p44/42, JNK, p38, and Akt kinases as assessed by phosphorylation was analyzed by Western blotting. Phosphoproteins were detected with phospho-specific antibodies. Equal loading was confirmed with pan-specific antibodies. Data are representative of four independent experiments. B, densitometric analysis of A. Data are presented as means ± S.D.; n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.001. C, Western blot analysis of Smad3 phosphorylation in TGF-β1-stimulated HLF. Data are representative of four independent experiments. D, densitometric analysis of C. Data are presented as means ± S.D.; n = 4; *, p < 0.05; **, p < 0.01. E, TGF-β1-dependent translocation of phospho-Smad3 (Ser-423/Ser-425) into the nucleus as assessed by immunofluorescence. HLF were incubated with TGF-β1 for 1 h and then washed, fixed, and stained with phospho-Smad3 (Ser-423/Ser-425) antibody. Arrows indicate nuclear localization of Smad3. Original magnification was ×40/1.25–0.75 oil objective. Bar size, 10 μm. Data are representative of three independent experiments. F, Western blot analysis of TGF-β1-driven translocation of phospho-Smad3 (Ser-423/Ser-425) into the nucleus. HLF were unstimulated or treated for 1 h with TGF-β1; nuclear extracts were prepared and immunoblotted with antibodies against phospho-Smad3, lamin B, and tubulin. Lamin B was used as a loading control, and tubulin was used to assess the purity of the nuclear fraction. Data are representative of three independent experiments.