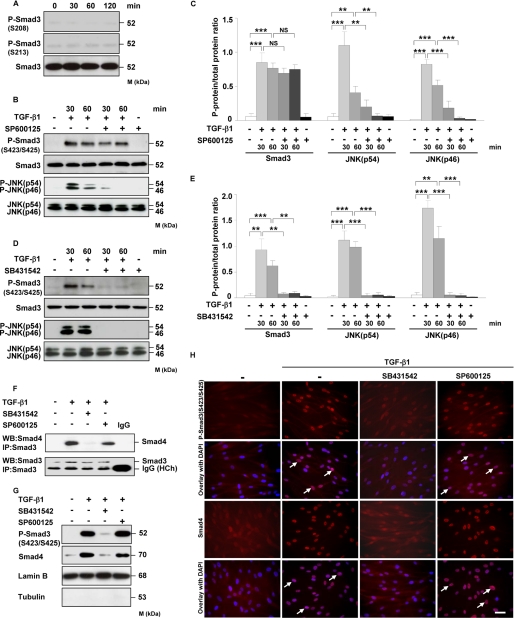
FIGURE 5.
JNK does not regulate Smad3 phosphorylation, association with Smad4, and its translocation into the nucleus. A, HLF were treated for the indicated time points with TGF-β1, and the phosphorylation of Smad3 at Ser-208 and Ser-213 was examined by Western blotting. Equal loading was confirmed with pan-specific antibody. Data are representative of four independent experiments. B and D, HLF were treated with TGF-β1 in the absence or presence of JNK inhibitor (SP600125) (B) or TβRI inhibitor (SB431542) (D) as indicated, and the activation of Smad3 and JNK as assessed by phosphorylation was analyzed by Western blotting. Data are representative of four independent experiments. C and E, densitometric analysis of B and D, respectively. Data are presented as mean ± S.D.; n = 4; **, p < 0.01; ***, p < 0.001. F, HLF were preincubated with SB431542 or SP600125 1 h prior to addition of TGF-β1. After 1 h, the cell lysates were prepared and immunoprecipitated (IP) with anti-Smad3 antibody. Coprecipitating Smad4 was detected by Western blotting (WB). The lower band present in the bottom panel represents a heavy chain (HCh) of anti-Smad3 antibody and isotype control. Data are representative of three independent experiments. G, Western blot analysis of TGF-β1-driven translocation of phospho-Smad3 (Ser-423/Ser-425) and Smad4 into the nucleus. HLF were pretreated with SB431542 or SP600125 and then either not stimulated or stimulated for 1 h with TGF-β1. Nuclear extracts were prepared and immunoblotted with antibodies against phospho-Smad3 (SER-423/Ser-425), Smad4, lamin B, and tubulin. Lamin B was used as a loading control, and tubulin was used to assess the purity of the nuclear fraction. Data are representative of three independent experiments. H, TGF-β1-driven translocation of phospho-Smad3 (Ser-423/Ser-425) and Smad4 into the nucleus as assessed by immunofluorescence. HLF were preincubated with SB431542 or SP600125 1 h prior to addition of TGF-β1. After 1 h, the cells were washed, fixed, and stained with phospho-Smad3 (Ser-423/Ser-425) and Smad4 antibodies. Arrows indicate nuclear localization of Smad3 and Smad4. Original magnification was ×40/1.25–0.75 oil objective. Bar size, 10 μm. Data are representative of three independent experiments.