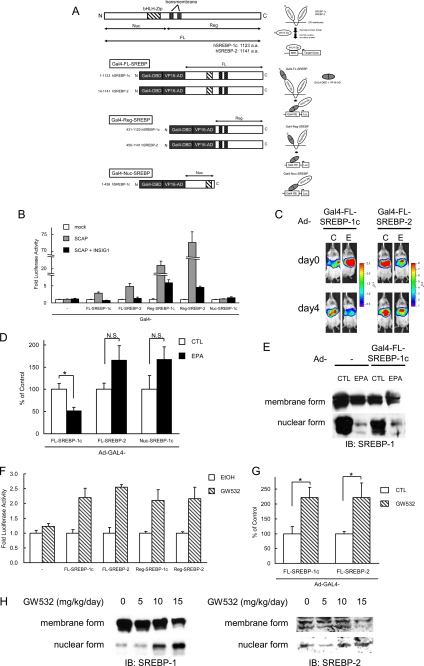
FIGURE 5.
PUFA suppresses the proteolytic activation of SREBP-1, not affecting SREBP-2. A, various regions of human SREBP-1c (amino acids (a.a.) 1–1123 (FL), 431–1123 (Reg), 1–436 (Nuc)) and human SREBP-2 (amino acids 14–1141 (FL), 450–1141 (Reg)) were fused to Gal4-DNA binding domain and VP16-transactivation domain. FL, full length; Nuc, nuclear; Reg, regulatory; bHLH-Zip, basic helix-loop-helix leucine zipper. GAL4-VP16-SREBP fusion protein is attached to the endoplasmic reticulum (ER) similarly as endogenous SREBP protein. Upon activation, its N-terminal region is released from the membrane into the nucleus by a cleavage process. The GAL4-VP16 promotes the luciferase (Luc) reporter gene expression by binding to GAL4-RE containing eight copies of upstream activation sequence, Gal4-binding site. B, regulation of cleavage of GAL4-VP16 fusion SREBP protein by SCAP and INSIG-1. HEK293 cells in a 48-well plate were co-transfected with GAL4-RE-Luc plasmid (100 ng/well), expression plasmids of GAL4-VP16 fusion SREBP protein (100 ng/well), SCAP (250 ng/well), INSIG-1 (100 ng/well), and pSV40-Renilla plasmid (50 ng/well). Cells were harvested 24 h after transfection. The firefly luciferase activity was measured and normalized by Renilla luciferase activity. All experiments were performed in triplicate. C–E, regulation of cleavage of GAL4-VP16 fusion SREBP protein by EPA in vivo. GAL4-RE-Luc adenovirus (Ad-GAL4-RE-Luc, 2.0 × 108 pfu/body) plus adenovirus expressing GAL4-VP16 fusion SREBP protein (Ad-Gal4-FL-SREBP-1c, Ad-Gal4-FL-SREBP-2 or Ad-Gal4-Nuc-SREBP-1c, 1.0 × 108 pfu/body) were intravenously injected into ICR male mice. After 2 days, the mice (n = 7–9 for each group) were treated orally with 7.5 g/kg EPA or water (CTL) once a day for 4 days. C, 0 and 4 days after the fast treatment of EPA, luciferin was injected intraperitoneally, and the luminescence from liver was captured with IVIS. The color overlay on the image represents the photons/s emitted from the animal with a range of 4.0 × 105–2.5 × 106 photons/s (Ad-Gal4-FL-SREBP-1c) and 1.0 × 106–8.0 × 106 photons/s (Ad-Gal4-FL-SREBP-2), as indicated by the color scale next to the images. D, quantification of luciferase activity with LivingImage software. Fold changes of luciferase activity on day 4 versus day 0 are shown. E, immunoblot analysis of mature and precursor SREBP-1 proteins from livers. Aliquots of nuclear extracts (10 μg) and total proteins (50 μg) from livers pooled equally from male mice for each group were subjected to immunoblot analysis. The primary antibodies used were polyclonal anti-mouse SREBP-1. N.S., not significant. F–H, SCAP ligand GW532 accelerates SREBP-1 cleavage. F, regulation of cleavage of GAL4-VP16 fusion SREBP protein by GW532. HEK293 cells in a 48-well plate were co-transfected with GAL4-RE-Luc plasmid (100 ng/well), expression plasmids for GAL4-VP16 fusion SREBP protein (100 ng/well), and pSV40-Renilla plasmid (50 ng/well). 3 h after transfection, GW532 (1 μm) or EtOH was added to media, and cells were harvested 24 h after transfection. The firefly luciferase activity was measured and normalized by Renilla luciferase activity. All experiments were performed in triplicate. G and H, GAL4-RE-Luc adenovirus (Ad-GAL4-RE-Luc, 2.0 × 108 pfu/body) plus adenovirus expressing GAL4-VP16 fusion SREBPs protein (Ad-Gal4-FL-SREBP-1c or Ad-Gal4-FL-SREBP-2, 1.0 × 108 pfu/body) were intravenously injected into ICR male mice. After 2 days, the mice (n = 8 for each group) were administered orally with GW532 or vehicle at indicated doses. 6 h after the last treatment, luciferin was injected intraperitoneally, and the luminescence from liver was captured with IVIS. G, quantification of luciferase activity with LivingImage software. Fold changes on day 3 versus day 0 are shown. H, immunoblot analysis of mature and precursor SREBP-1 and SREBP-2 proteins from livers. Aliquots of nuclear extracts (10 μg) and total proteins (50 μg) from livers pooled equally from four male mice for each group were subjected to immunoblot (IB) analysis. The primary antibodies used were polyclonal anti-mouse SREBP-1 and anti-mouse SREBP-2. These data are representative of at least two independent experiments (n = 3–8 mice/group). Results are means ± S.E. *, p < 0.05.