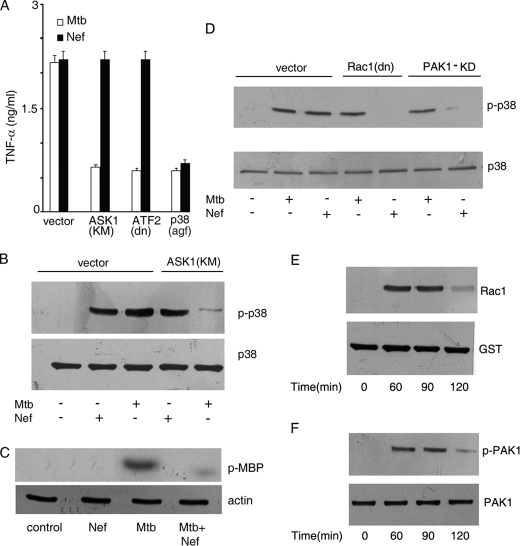
FIGURE 2.
Nef inhibits M. tuberculosis-mediated activation of p38 MAPK and ASK1. A, THP-1 cells were transfected with vector alone or with a kinase-dead mutant of ASK1 (ASK1(KM)) or dominant-negative ATF2 (ATF2(dn)) or dominant-negative p38 MAPK (p38(agf)). Transfected cells were treated with M. tuberculosis (Mtb) or Nef separately, and release of TNF-α was measured as described under Fig. 1A. Data represent the means ± S.D. of three separate experiments. B–D, THP-1 cells transfected with either empty vector or ASK1 (KM) (B) or Rac1 (dn) (D) or PAK1-KD (D) were left untreated or treated with Mtb or with Nef separately for 90 (B and D) or 60 (C) min. Cell lysates were either immunoblotted with phospho-p38 MAPK antibody and reprobed with p38 MAPK antibody (B and D) or immunoprecipitated with ASK1 antibody, and the immunoprecipitate was used to study the phosphorylation of myelin basic protein using [γ-32P]ATP followed by autoradiography (C). Actin in the cell lysate was blotted to confirm equal amounts of proteins in cell lysates (bottom panel of C). E, activation of Rac1 in cellular extracts was assessed by affinity precipitation of the Rac1-GTP complex from whole cell lysates using PAK1-PBD followed by Western blotting using anti-Rac1 antibody as described under “Experimental Procedures.” F, cell extracts were prepared, and phosphorylation of PAK1 was assessed by Western blotting using anti-phospho-PAK1 antibody followed by reprobing with PAK1 antibody, respectively. The data in panels B–F are representative of those obtained in three different experiments.