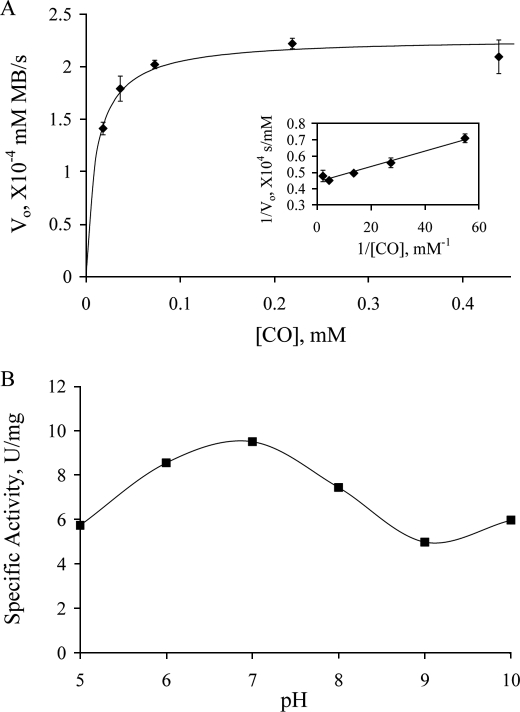
FIGURE 3.
Steady-state kinetics of CODH reaction with CO using methylene blue as the electron acceptor. A, plot of initial rate (following the absorbance decrease at 615 nm upon reduction of methylene blue, after mixing 4.4 nm CODH with CO-dissolved (10–438 μm) methylene blue) as a function of [CO] in 50 mm potassium phosphate, pH 7.2, 25 °C. The solid line is the fit with the Michaelis-Menten equation using kinetic parameters obtained from the double-reciprocal plot (inset). B, the pH dependence of CODH activity, with specific activity given in units/mg, and 1 unit is 1 μmol of CO oxidized/min. Buffers used in the experiments are: MES pH 5.5, potassium phosphate pH 6 and 7, Tris pH 8, CHES pH 9, and CAPS pH 10, all at 50 mm.