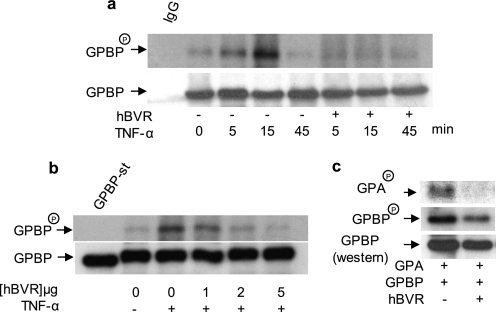
FIGURE 2.
hBVR suppresses the kinase activity of GPBP. a, TNF-α-stimulated phosphorylation of GPBP is suppressed by hBVR. HEK293A cells transfected with GPBP alone or co-transfected with hBVR-pcDNA expression plasmids were metabolically labeled with [32P]H3PO4. Cell lysates were obtained, and GPBP was immunoprecipitated. The precipitate was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and autoradiographed. The blot was subsequently probed with anti-GPBP antibody followed by ECL detection as the control for loading. Immunoprecipitates with mouse IgG and anti-mouse IgG-ECL was used as the control for specificity of binding. b, hBVR decreases GPBP autophosphorylation in a concentration-dependent manner. Cells were transfected with FLAG-GPBP expression plasmid together with increasing concentrations of pcDNA3-hBVR. One day later cells were starved overnight and thereafter treated with TNF-α (20 ng/ml, 15 min). GPBP was isolated with anti-FLAG beads and assayed in the kinase reaction as described under “Experimental Procedures” (6). c, hBVR suppresses GPBP-dependent phosphorylation of its substrate, the GPA-derived peptide. Cells were transfected with expression plasmid for FLAG-GPBP alone or co-transfected with pcDNA3-hBVR followed 24 h later by starvation overnight, treatment with TNF-α as in b, and subsequent lysis. GPBP was isolated from the lysates using anti-FLAG beads. The N-terminal peptide of GPA was isolated as a GST fusion polypeptide from E. coli and used as the substrate in the GPBP kinase reaction assay. The reaction mixture was resolved by SDS-PAGE and transferred to nitrocellulose membrane, and phosphorylated GPBP and GPA (GPBPP and GPAP) was visualized by autoradiography. The membrane was subsequently probed with anti-GPBP antibody followed by ECL detection.