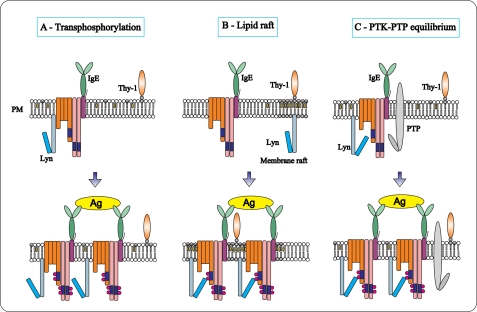
FIGURE 11.
Models of the initial tyrosine phosphorylation of the FcϵRI. A, according to the “transphosphorylation model,” a fraction of the tetrameric FcϵRI (αβ2γ) in the plasma membrane (PM) is associated with Lyn kinase. After binding of the IgE to the FcϵRI α subunit, the receptor remains randomly distributed at the plasma membrane. Stimulation with bivalent or multivalent antigen (Ag) leads to receptor dimerization or multimerization. Only in the clusters can the Lyn kinases phosphorylate ITAMs of the neighboring receptors. Monovalent ligands do not cluster the receptors and therefore do not induce phosphorylation and activation. B, “lipid raft model” proposes that Lyn kinase is not bound to FcϵRI but is separated instead into membrane regions (lipid rafts), which are enriched with cholesterol and glycosphingolipids. These domains possess some other lipid-anchored molecules, like glycosylphosphatidylinositol-anchored Thy-1 glycoprotein. After antigen-mediated FcϵRI clustering, the receptors are recruited into lipid rafts where their ITAMs are phosphorylated by Lyn. C, according to “PTK-PTP equilibrium model,” a fraction of Lyn kinase in resting cells is associated with FcϵRI and phosphorylates it. This activity is counterbalanced by the action of PTPs, resulting in the formation of a basal equilibrium in quiescent cells. Perturbation of this equilibrium by inhibiting the enzymatic activity of the PTPs or by clustering-induced allosteric changes in the receptor shifts the balance between phosphorylation and dephosphorylation resulting in a net increase in tyrosine phosphorylation of the receptor.