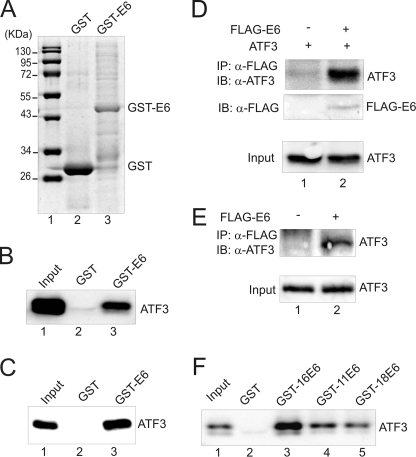
FIGURE 1.
ATF3 directly binds to E6. A, GST-E6 and GST proteins eluted from glutathione-agarose were subjected to SDS-PAGE and stained with Coomassie Blue. B, immobilized GST-E6 or GST protein was incubated with in vitro-translated ATF3 protein at 4 °C overnight. After extensive washes, bound proteins were eluted and subjected to immunoblotting with an ATF3 antibody. Lane 1 represents 10% of total input protein. C, immobilized GST-E6 or GST was incubated with 200 ng of purified ATF3 protein followed by immunoblotting to visualize bound proteins. The input lane represents 10% of total input protein. D, ATF3 was expressed in H1299 cells with or without FLAG-E6 by transfections. Cell lysates were immunoprecipitated by agarose conjugated with an anti-FLAG antibody, and bound proteins were eluted for immunoblotting as indicated. E, HCT116 p53−/− cells were transfected with the plasmid encoding FLAG-E6. After treated with 25 μm MG132, cells were subjected to immunoprecipitations as in D to examine the interaction of FLAG-E6 with the endogenous ATF3 protein. F, GST-E6 proteins derived from HPV11 (GST-11E6), HPV16 (GST-16E6), and HPV18 (GST-18E6) were immobilized on glutathione-agarose, and incubated with in vitro-translated ATF3 proteins for GST-pulldown assays as in B.