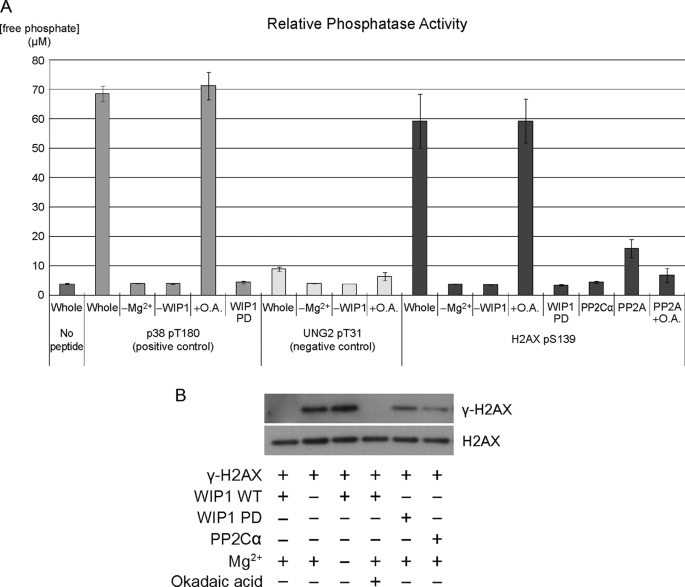
FIGURE 1.
γ-H2AX is dephosphorylated by WIP1 in vitro. A, H2AX pS139 phosphopeptide was incubated with human recombinant WIP1 protein. p38 pT180 and UNG2 pT31 phosphopeptides were used as a positive and negative control, respectively. Free phosphate released from the phosphopeptide was measured by malachite green phosphate assay to determine relative phosphatase activities on each phosphopeptide. Error bars correspond to standard error (n = 3). O.A., okadaic acid. WIP1 PD is a phosphatase-dead point mutant used as a control. B, 293T cells were transfected with myc-H2AX expression plasmid and treated with bleomycin for 1 h to induce γ-H2AX phosphorylation. Myc-tagged full-length H2AX protein was immunopurified using anti-Myc antibody and incubated with human recombinant WIP1 protein, WIP1 phosphatase-dead protein, or PP2C alpha in vitro. γ-H2AX phosphorylation levels were determined by immunoblotting using a γ-H2AX-specific antibody. The key components of each assay are indicated below each lane.