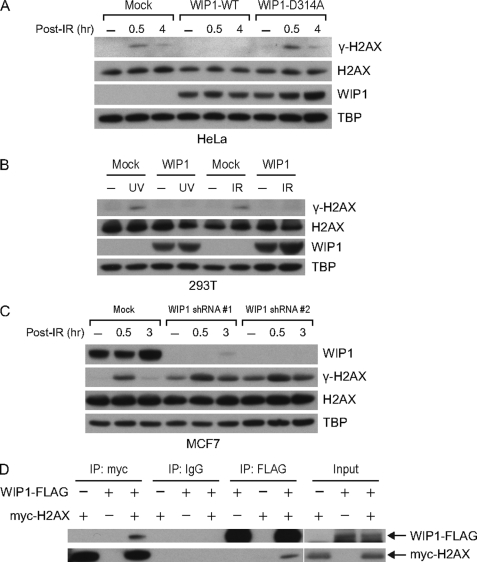
FIGURE 2.
WIP1 impairs γ-H2AX phosphorylation in cells. A, overexpression of WIP1 suppresses γ-H2AX in IR-treated HeLa cells. HeLa cells were either mock transfected or transfected with WIP1-FLAG expression plasmid. At 24 h after transfection, cells were irradiated with 5-Gy IR and harvested. Nuclear lysates were immunoblotted to assess γ-H2AX and total H2AX protein, and WIP1 protein. TBP (TATA-binding protein) served as a loading control. B, overexpression of WIP1 suppresses γ-H2AX in IR and UV-treated 293T cells. 293T cells were transfected as in A. After 24 h, cells were irradiated with either 5-Gy IR or 50 J/m2 UV and harvested 30 min after IR or 2 h after UV treatment. Nuclear lysates were immunoblotted as indicated. C, WIP1 knockdown increased γ-H2AX levels in IR-treated MCF7 cells. Cells were transduced with lentivirus expressing WIP1 shRNA (#1 and #2). After 48 h, cells were irradiated with 5-Gy IR, harvested, and then analyzed by immunoblot with the indicated antibodies. D, WIP1 and H2AX physically interact in 293T cells. 293T cells were co-transfected with myc-H2AX and WIP1-FLAG expression plasmids. Interaction between H2AX and WIP1 was examined by immunoprecipitation with anti-FLAG antibody followed by immunoblotting with anti-Myc antibody and vice versa.