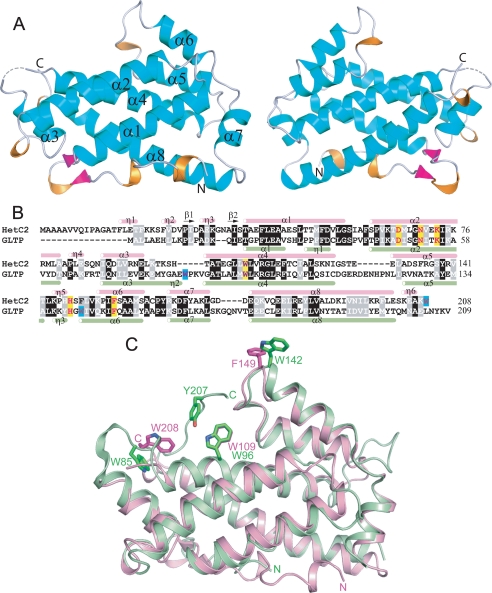
FIGURE 1.
A, tertiary structure of HET-C2 in the apo-form. Front and back views of HET-C2 are shown with α-helices (cyan), 310 helices (orange), β-sheet (magenta), and loop segments (light gray) in a schematic diagram. B, structure-based sequence alignment between HET-C2 and human GLTP. Secondary structural elements of HET-C2 (pink) and GLTP (light green) are shown above and below the aligned sequences by tubes indicating 310 (η) and α-helices (α) and arrows indicating β-strands (β). Conserved residues that interact with the ceramide-linked sugar of glycolipids are highlighted in yellow in GLTP and HET-C2. Nonbinding site Trp residues are highlighted in turquoise. C, structural superposition of HET-C2 (pink) and human GLTP (green). Key aromatic residues are shown in stick representation. Trp142 of GLTP and Phe149 of HET-C2 are localized on the surface and are completely accessible to the aqueous milieu. Human apoGLTP structure was solved previously (9, 10).