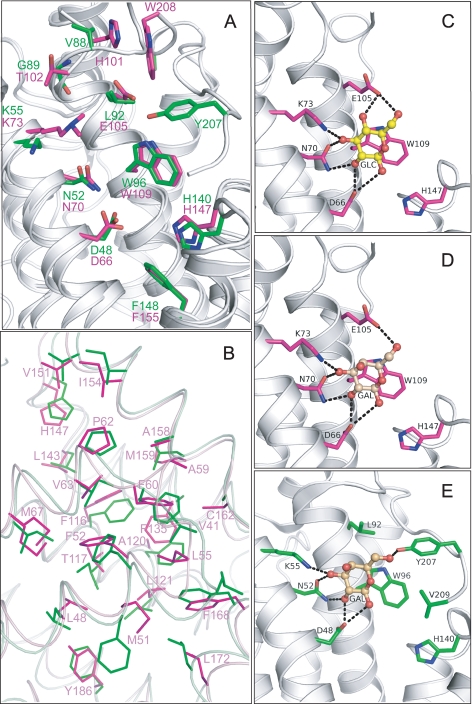
FIGURE 2.
A, structural superposition of HET-C2 (magenta) and GLTP (green) showing conserved residues present in the pocket that interacts with the sugar-amide moiety of glycolipids. Lys73 in HET-C2 is shown containing the exogenous dimethyl group introduced to facilitate crystallization. Human apoGLTP structure was solved previously (9, 10). B, residue homology and similarity between hydrophobic pockets of HET-C2 and human GLTP. For clarity, only HET-C2 residues have been labeled. The following positional correspondence is observed for HET-C2 and GLTP, respectively: Leu48 = Leu30, Met51 ≈ Phe33, Phe52 = Phe34, Leu55 = Leu37, Ala59 ≈ Val41, Phe60 = Phe42, Pro62 = Pro44, Val63 ≈ Ile45, Met67 ≈ Ile49, Phe116 = Phe103, Thr117 ≈ Ile104, Ala120 ≈ Phe107, Leu121 = Leu108, Phe135 ≈ Ala128, Leu143 = Leu136, His147 = His140, Val151 = Val144, Ile154 = Ile147, Ala158 = Ala151, Met159 ≈ Leu152, Cys162 ≈ Ala155, Phe168 = Phe161, Leu172 = Leu165, Tyr186 ≈ Phe183. C, docking of Glc onto the GSL headgroup binding site of HET-C2. Side chains of HET-C2 residues involved in Glc binding are shown in magenta. The Glc sugar ring is colored yellow with red balls representing oxygen. The black dotted lines represent hydrogen bonds. To provide spatial relationships reflecting the situation in wild type HET-C2, the exogenous dimethyl group added to Lys73 to facilitate HET-C2 crystallization is not shown. D, docking of Gal onto the GSL headgroup binding site of HET-C2. The color scheme is the same as for C except that the galactose sugar ring is colored beige. E, docking of galactose onto the GSL headgroup binding site of human GLTP. The color scheme is the same as for D except that the side chains of GLTP residues involved in Gal binding are shown in green. The galactose location is derived from Protein Data Bank entry 2EVL (10).