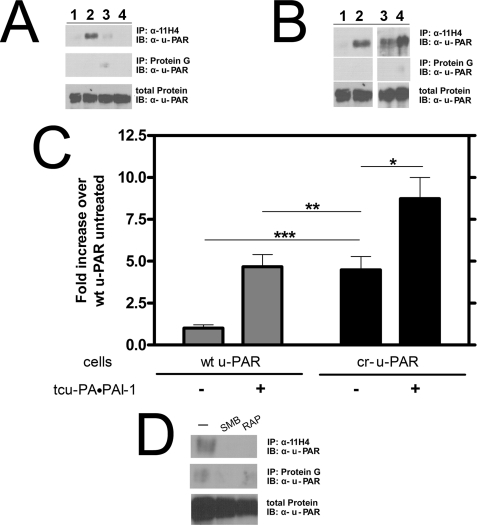
FIGURE 6.
cr-u-PAR co-immunoprecipitates with LRP in the absence of tcu-PA·PAI-1 complexes. A, cells expressing wt u-PAR were incubated with buffer (lane 1), 10 nm tcu-PA·PAI-1 (lane 2), 500 nm RAP (lane 3), or 500 nm RAP, and 10 nm tcu-PA·PAI-1 complex (lane 4). u-PAR was affinity-precipitated with the monoclonal α-LRP antibody, 11H4, and detected using polyclonal α-u-PAR antibody. Nonspecific binding of u-PAR to protein G beads was minimal. B, cells expressing wt u-PAR (lanes 1 and 2) or cr-u-PAR (lanes 3 and 4) were incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 10 nm tcu-PA·PAI-1 complex and u-PAR affinity-precipitated as described under “Experimental Procedures.” The band intensities are shown (C), with data representing the mean ± S.D. of three individual replicates (t test; *, p < 0.03; **, not significant; and ***, p < 0.0005). D, cr-u-PAR-expressing cells were treated with buffer only (lane 1), 4 μm SMB (lane 2), or 500 nm RAP (lane 3) for 30 min before lysis and immunoprecipitation with α-LRP antibody followed by immunoblotting with α-u-PAR antibody.