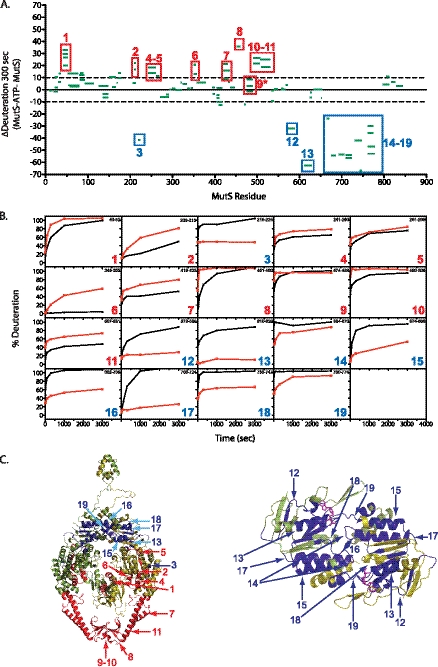
FIGURE 3.
ATPγS alters solvent accessibility for regions of MutS. A, change in percent deuteration of individual peptides from MutS in the presence of 250 μm ATPγS and MutS at 300 s. Peptides measured in both samples are shown as green bars spanning over the indicated sequence on the x axis. Negative and positive y axis values indicate increased protection and increased solvent accessibility, respectively, caused by addition ATPγS. Regions of substantial change upon ATPγS addition are boxed. B, kinetics of deuteron incorporation for a representative peptide of MutS in the presence (red) and absence (black) of ATPγS for each region boxed in A. C, regions of solvent protection (blue) and increased solvent accessibility (red) upon ATPγS addition are mapped onto the ribbon diagram of MutS.