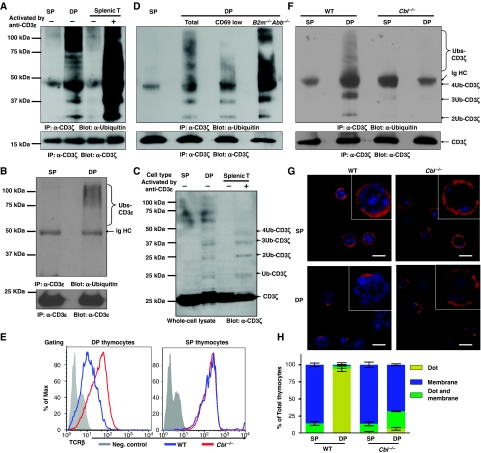
Figure 1.
c-Cbl-mediated tonic ubiquitylation of CD3 on immature T cells. (A–D, F) DP and SP thymocytes were purified by FACS, and splenic T cells purified by MACS. Splenic T cells were either resting or activated by crosslinking CD3ɛ for 2 min. (A) SP thymocytes (3 × 107), DP thymocytes (24 × 107), and resting or activated splenic T cells (3 × 107) were lysed in 1 ml lysis buffer. The lysates were immunoprecipitated with CD3ɛ antisera (551ζ), separated by SDS–PAGE and western blots was probed with Ub mAb (P4D1). Blots were stripped and reprobed with CD3ζ mAb (H146) to show that an equal amount of CD3ζ was analysed in each sample. (B) After immunoprecipitation with CD3ζ mAb as described in (A), the lysates were further immunoprecipitated with CD3ɛ mAb (2C11), separated by SDS–PAGE and western blots was probed with Ub mAb. Blots were stripped and reprobed with CD3ɛ mAb (HTM3.1). (C) Lysates (20 μl) were separated by SDS–PAGE and immunoblotted with CD3ζ mAb. (D, F) Analysis was performed as in (A), except that wild-type CD69low and B2m−/−Abb−/− [MHC class I and class II deficient] DP thymocytes (D), or c-Cbl−/− SP and DP thymocytes (F) were purified by FACS and included in the analysis. (E) DP and SP thymocytes were purified from either C57BL/6J mice or c-Cbl−/− mice, and TCRβ surface expression was measured by flow cytometry. (G, H) Thymocytes were stained with mAbs against CD4 and CD8, and DP and SP thymocytes purified by FACS. Sorted thymocytes were fixed, permeabilized, and stained with Alexa-647-conjugated CD3ζ mAb. The localization of CD3ζ (red) and DAPI-stained nucleus (blue) are shown in representative confocal images (G). The distribution of CD3ζ in thymocyte populations is indicated (n>50) (H).