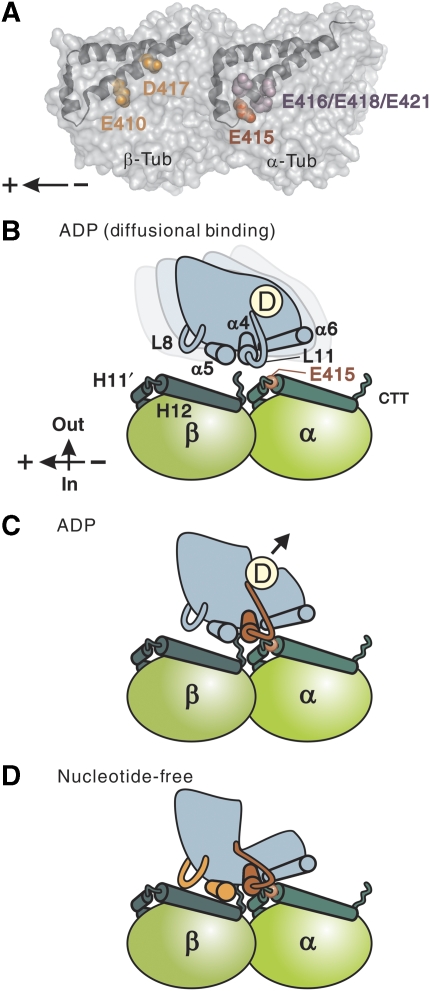
Figure 6.
Structural basis for MT-dependent activation of kinesin ATPase. (A) Two independent sites on the MT critical for kinesin motility: the negatively charged residues spanning the H11–12 loop and H12 of α-tubulin (highlighted in red and purple), and the negatively charged residues in H12 of β-tubulin (orange). A tubulin dimer is viewed from the putative outside of the MT tube with its minus end to the right. (B–D) Schematic model for kinesin interaction with MTs in different nucleotide states. In the diffusional binding state (B), kinesin, tightly holding ADP, is in search of the next binding site through diffusion along the MT. Nonspecific electrostatic interaction between the negatively charged C-terminal tail of tubulin (CTT) and the positively charged interface of kinesin contributes to this weak interaction. When the switch II helix/loop of kinesin (red) encounters the H11–12 loop/H12 of α-tubulin (C), kinesin changes its conformation and ejects ADP from the nucleotide pocket. After, or concomitant with ADP release (D), kinesin is further locked on the MT through the interaction between L8/α5 of kinesin (orange) and H12 of β-tubulin. This stable interaction enables kinesin to sustain load.