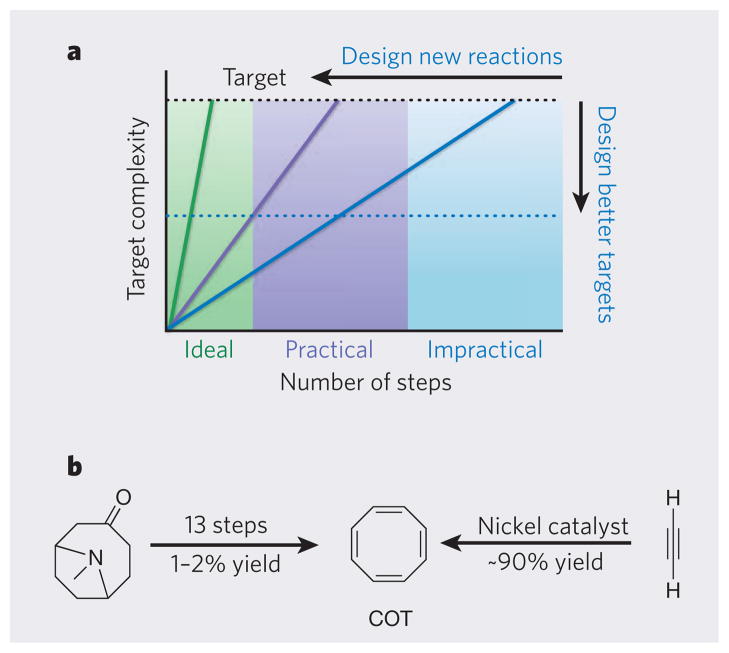
Figure 4. Developing ideal syntheses.
a, In an ideal synthesis (green line), a single step converts simple reactants into a structurally complex product. The syntheses of complex natural products often require too many steps (blue line), rendering them impractical for making large quantities of product. Reactions that generate a large increase in structural complexity per step allow shorter, more practical syntheses to be developed (purple line). For natural products that have valuable functional properties, another way to achieve step economy is to target structurally simpler compounds (blue dotted line) that have the same (or better) biological properties than the natural product. b, The first published synthesis of cyclooctatetraene (COT) required 13 steps, and the overall yield was very low. The discovery of a nickel-catalysed reaction allowed COT to be made in 90% yield in one step.