Chart 2.
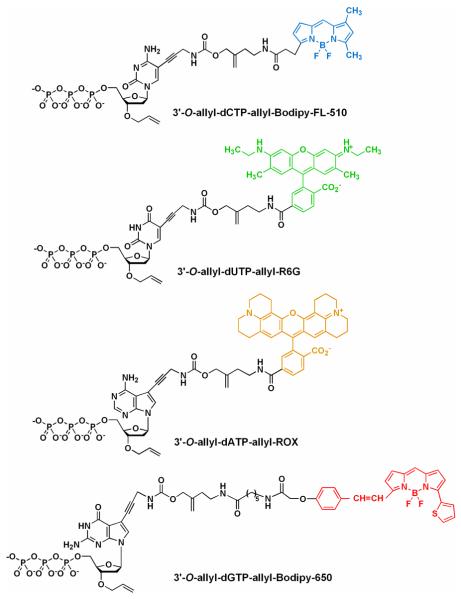
Structures of an example of G-1 modified nucleotides (3′-O-ally-dNTP-allyl-fluorophores), with the 4 fluorophores having distinct fluorescent emissions: 3′-O-allyl-dCTPallyl-bodipy-FL-510 [λabs(max) = 502 nm; λem(max) = 510 nm], 3′-O-allyl-dUTP-allyl-R6G [λabs(max) = 525 nm; λem(max) = 550 nm], 3′-O-allyl-dATP-allyl-ROX [λabs(max) = 585 nm; λem(max)= 602 nm], and 3′-O-allyl-dGTP-allyl-bodipy-650 [λabs(max) = 649 nm; λem(max) = 670 nm].