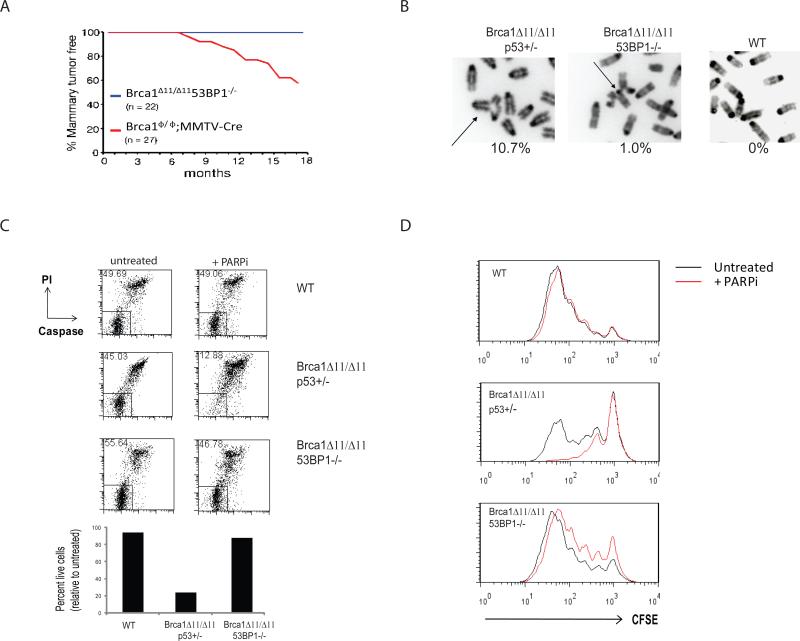
Figure 1. Deletion of 53BP1 reduces mammary tumorigenesis, radial chromosome formation and cellular proliferation defects in Brca1Δ11/Δ11 cells.
(A) Breast cancer incidence in mice deficient in Brca1. The red line indicates tumor incidence in mice with deletion of Brca1 exon 11 targeted to the mammary cells using a Cre transgene under control of the MMTV LTR promoter (Brca1ϕ/ϕ;MMTV-Cre). The blue line shows mammary tumor incidence in Brca1Δ11/Δ11 53BP1-/- mice, in which Brca1 exon 11 and 53BP1 are deleted in all cells. (B) Radial chromosome structures characteristic of metaphases from Brca1Δ11/Δ11 p53+/- B cells. The percentage of metaphases containing radial chromosomes in Brca1Δ11/Δ11 p53+/- (n=300), Brca1Δ11/Δ11 53BP1-/- (n=300) and WT (n=100) cells is indicated. Note that equivalent results were seen with Brca1Δ11/Δ11 p53+/- and Brca1Δ11/Δ11 p53-/-cells. (C) Flow cytometry analysis of B cells to measure cell survival. The labeled populations in the dot plots are viable cells, identified by their ability to exclude PI and their lack of caspase 3 activation. The chart shows the frequency of live cells treated with PARP inhibitor normalized to the untreated population. (D) Proliferation of B cells pulsed with CFSE and cultured with and without PARP inhibitor. CFSE signal diminishes with increasing cell division, so that cells that do not divide have the highest CFSE signal. See also Fig. S1.