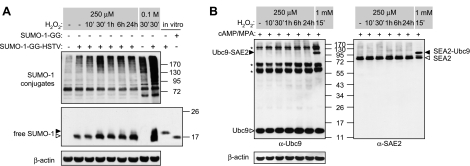
Figure 2.
Modest levels of ROS do not trigger oxidative modifications of SUMO enzymes. A) HESCs were transfected with full-length immature SUMO-1 (SUMO-1-GG-HSTV) (500 ng), and at 24 h post-transfection were treated with 250 μM H2O2 for the indicated times. Some cultures were exposed for 30 min to a concentration of 0.1 M H2O2, known to inactivate SUMO proteases. Whole-cell protein extracts were resolved on a 7.5% SDS-polyacrylamide gel and immunoprobed with anti-SUMO-1 antibody to visualize SUMO-1 conjugates. For detection of free SUMO-1, samples were separated on a 12% gel. In vitro expression using vectors encoding the immature (SUMO-1-GG-HSTV) and matured form (SUMO-1-GG) of SUMO-1 were used as positive controls. Open arrowhead indicates the position of C-terminally processed SUMO-1; solid arrowhead indicates the immature form. B) HESCs were decidualized with 8-br-cAMP and MPA for 3 d and subjected to time-course treatment with 250 μM H2O2 over a 24-h period. As a positive control for Ubc9-SAE2 cross-linking, some cultures were treated with 1 mM H2O2 for 15 min. Total protein harvested in Laemmli buffer not containing β-mercaptoethanol was analyzed by Western blotting with anti-Ubc9, SAE2, and β-actin antibodies. Open arrowheads indicate positions of the uncoupled proteins; solid arrowheads show Ubc9-SAE2 cross-linked species; asterisks indicate nonspecific bands detected by the anti-Ubc9 antibody.