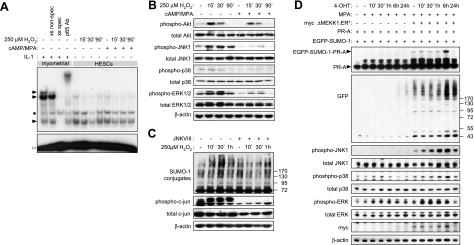
Figure 3.
JNK signaling mediates ROS-dependent hypersumoylation. A) Undifferentiated and decidualizing primary HESCs were exposed to 250 μM H2O2 for the indicated times, and DNA binding of NF-κB contained in the nuclear extracts was analyzed by EMSA. Nuclear lysate from myometrial cells stimulated with 1 ng/ml IL-1β for 30 min was used as a positive control. Myometrial lysates were also incubated with 100-fold excess of unlabeled NF-κB (xs spec) and Oct1 (xs nonspec) oligonucleotides prior to the addition of the [32P]-labeled probe, as DNA binding competitors to verify binding specificity. For supershift analysis, myometrial nuclear extracts were incubated with an antibody against p65 prior to addition of the hot probe. Solid arrowheads indicate position of specific complexes; solid bullet indicates position of nonspecific complexes; open arrowhead indicates the position of uncomplexed DNA probe. B) Primary HESC cultures were kept undifferentiated or decidualized with 8-br-cAMP and MPA for 3 d and then exposed to 250 μM H2O2 for 0, 15, 30, or 90 min. Whole-cell extracts were subjected to Western blot analysis with antibodies against the total and phosphorylated forms of Akt, JNK, p38, and ERK1/2. C) Undifferentiated HESC cultures were either kept untreated or treated with 36 μM of JNKVIII inhibitor for 2 h before exposure to 250 μM H2O2 for 10, 30, or 60 min. Whole-cell extracts were subjected to Western blot analysis with antibodies against SUMO-1, total and phosphorylayed forms of c-jun, and β-actin as loading control. D) HeLa cells were transfected with PR-A (500 ng) and EGFP-SUMO-1 (50 ng), with or without ΔMEKK1:ER*-myc (500 ng). Twenty-four hours later, cells were treated with MPA and 4-hydroxytamoxifen (4-OHT) for the times indicated. Western blotting was performed on whole-cell protein extracts by using antibodies against PR, GFP, myc tag, β-actin, and total or phosphorylated forms of JNK, p38, and ERK1/2.