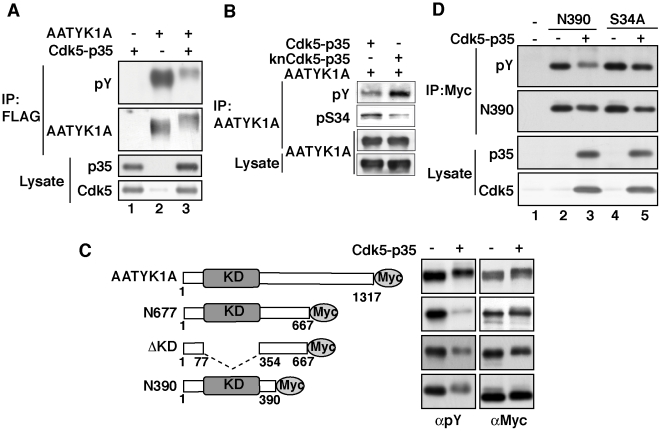
Figure 6. Phosphorylation of AATYK1A by Cdk5 suppressed its tyrosine phosphorylation.
(A) Effect of Cdk5/p35 on the tyrosine phosphorylation of AATYK1A. AATYK1A-Flag was transfected into HEK293 cells, alone or with Cdk5/p35. Twenty-four hours after transfection, cells were treated with 50 µM pervanadate for 20 min. AATYK1A was immunoprecipitated with the anti-Flag antibody and examined for tyrosine phosphorylation using immunoblotting with the anti-phospho-Tyr antibody (pY). (B) COS-7 cells expressing AATYK1A and Cdk5/p35 or knCdk5/p35 were treatment with 100 µM pervanadate for 5 min. After immunoprecipitation with the anti-AATYK1A antibody, their tyrosine phosphorylation was examined using the anti-phospho-Tyr antibody (pY) and Ser34 phosphorylation was detected in the immunoprecipitates with anti-pS34 antibody. (C) Tyrosine phosphorylation of AATYK1A and of its deletion mutants, N677, ΔKD, or N390. AATYK1A or its deletion mutants was transfected into COS-7 cells, alone (–) or with Cdk5/p35 (+). After treatment with 100 µM pervanadate for 5 min, the tyrosine phosphorylation of AATYK1A was examined after immunoprecipitation with the anti-Myc antibody. (D) N390 or N390-S34A (S34A) was expressed in COS-7 cells, alone (–) or with Cdk5/p35 (+). Tyrosine phosphorylation was induced by the pervanadate treatment at 100 µM for 5 min. After immunoprecipitation with the anti-Myc antibody, their tyrosine phosphorylation was examined using the anti-phospho-Tyr antibody (pY).