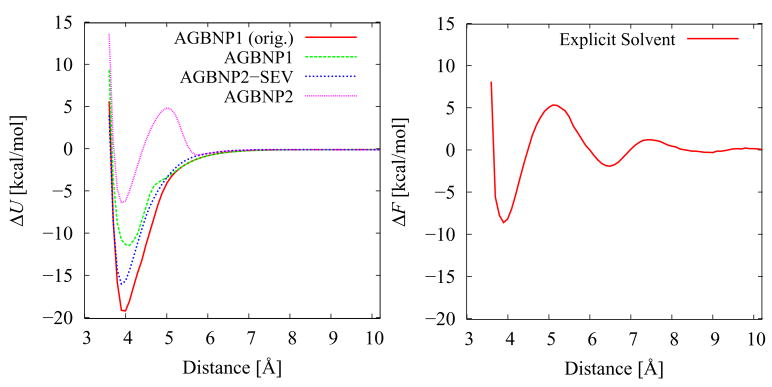
Figure 10.
Potential of mean force of ion pair formation between propyl-guanidinium and ethyl-acetate in the coplanar orientation with AGBNP implicit solvation (A) and explicit solvation (B; reference 130). In (A) “AGBNP1 (orig.)” refers to the original AGBNP1 parametrization,42 “AGBNP1” refers to the AGBNP1 model used in this work which includes a surface tension parameter correction for the carboxylate group aimed at reducing the occurrence of ion pairs,55 “AGBNP2” refers to the current model and “AGBNP2-SEV” the current model without hydrogen bonding and surface tension corrections. The potentials of mean force are plotted with respect to the distance between the atoms of the protein sidechain analogs equivalent to the Cζ of arginine and the Cγ of aspartate.