Figure 5.
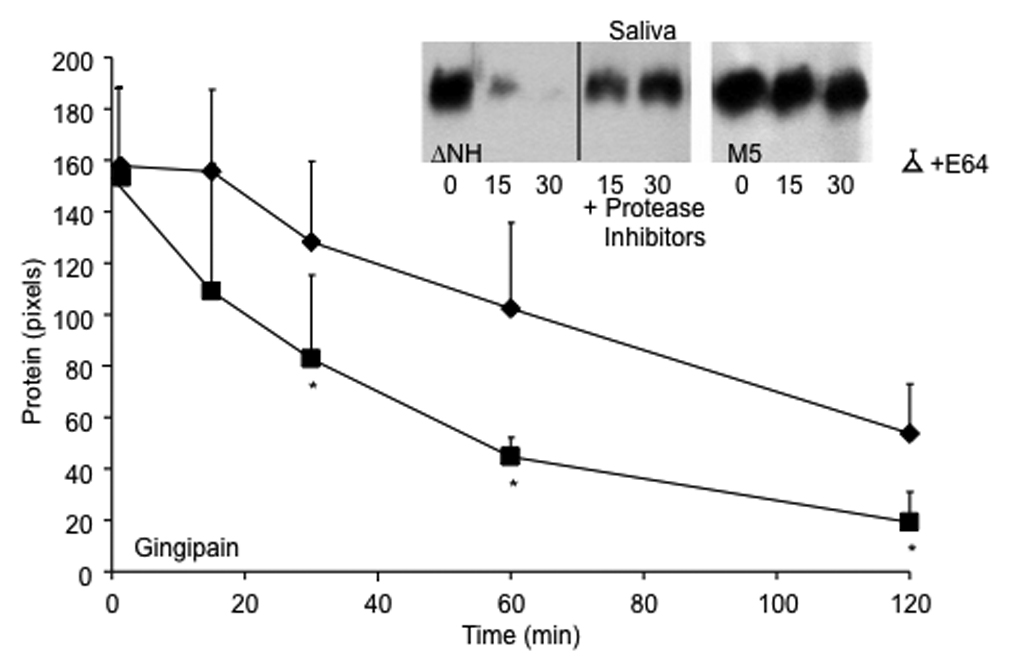
Degradation of M protein on the streptococcal surface by gingipain (line graph) and saliva (inset). M5 strain (diamonds) and its ΔNH2 mutant (squares) were grown overnight in THB containing E64, washed in appropriate buffers and exposed to gingipain or saliva for the indicated time periods at 37°C in the absence and presence of a cocktail of protease inhibitors. To estimate the amount of M5 protein remaining on the streptococcal surface, the hot acid extract of the treated bacteria was spotted onto Protan membrane with a BioRad Bio-Dot manifold, blocked and overlaid with anti-PepM5 antiserum. Bound antibodies were detected by Pico-west and densitometry was performed using NIH Image as described in Materials and Methods. The amount of M protein was expressed as mean pixels of three experiments +/−S.D. The mean value for extracts from bacteria treated for 120 minutes with gingipain in buffer devoid of cysteine and supplemented with protease inhibitors (open triangle) were not significantly different from those shown for untreated bacteria determined at time zero. Asterisks denote significant differences between M5 strain and its ΔNH2 mutant. Similar results were obtained by treating the M5 wt (right gel, inset) and ΔNH2 mutant (left gel, inset) with human saliva. Supernatants were electrophoresed under reducing conditions and blotted to detect M5 protein as described in material and methods.
