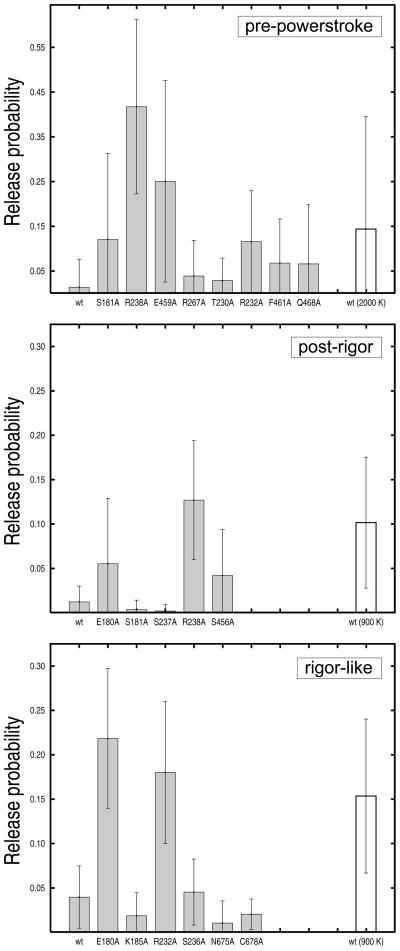
FIG. 5.
Release probabilities from pre-powerstroke, post-rigor, and rigor-like structures for the wild-type myosin and a series of single-point alanine mutants. The results were obtained from twenty MCES runs carried out in the pre-release temperature range; i.e., a ligand temperature of 1500 K was employed in the pre-powerstroke state and 600 K in the rigor-like and post-rigor states. Uncertainties were estimated from the standard deviation of the probability distributions. White boxes correspond to the release probability of the wild-type myosin in the post-release temperature range (i.e., a ligand temperature of 2000 K in the pre-powerstroke state and 900 K in the rigor-like and post-rigor states) and are used for comparison. The mutational studies show that: (i) alanine substitutions of the salt-bridging residues R238 and E459 significantly lower the release barrier in the pre-powerstroke state (top); (ii) the same is true for R238 in the post-rigor state (middle); (iii) mutations of residues E180 and R232 into alanines have large effects in the rigor-like but not in the pre-powerstroke state.