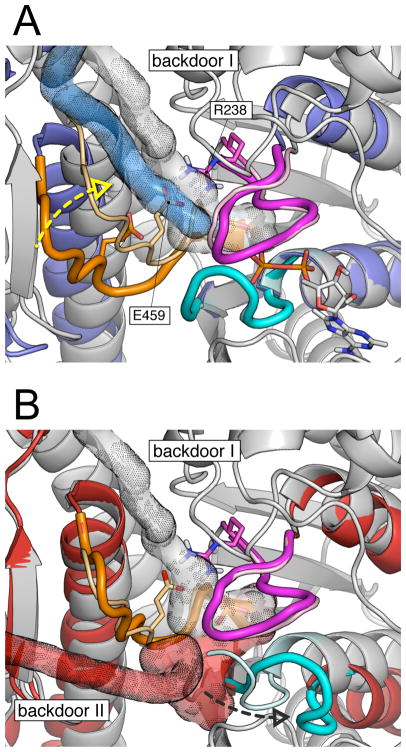
FIG. 6.
Comparison of Pi release paths in various myosin structures. (A) Pi release from the pre-powerstroke (grey) and the post-rigor (blue) states; the structures were aligned on the N-terminal subdomain. The simulation results indicate that in both cases Pi is released through backdoor I between the U50 and L50 subdomains. The exit path goes between switch I and switch II and mainly involves the salt-bridging residues R238 (part of switch I) and E459 (part of switch II). The result that the release barrier was found to be much higher in the pre-powerstroke state along with the mutagenesis data strongly indicate that R238 and E459 are crucial for Pi release through backdoor I. When switch II is open (i.e., post-rigor), the salt-bridge is not formed and the activation barrier is sensibly lower. Closing of switch II, while going from the post-rigor to the pre-powerstroke state (yellow dashed arrow), restores the salt-bridge and the activation barrier increases. (B) Pi release from the pre-powerstroke (grey) and the rigor-like (red) states; the structures were aligned on the L50 subdomain. In the rigor-like state Pi release proceeds through an alternative path, here named backdoor II. The latter involves residues of switch II and the P-loop and releases the phosphate through a tube located between the N-terminal and L50 subdomains. Backdoor II opening requires the displacement of the P-loop (black dashed arrow), which moves away from the switches. The P-loop movement does not involve the breaking of the salt-bridge between R238 and E459, which is distant from the rigor-like exit path.