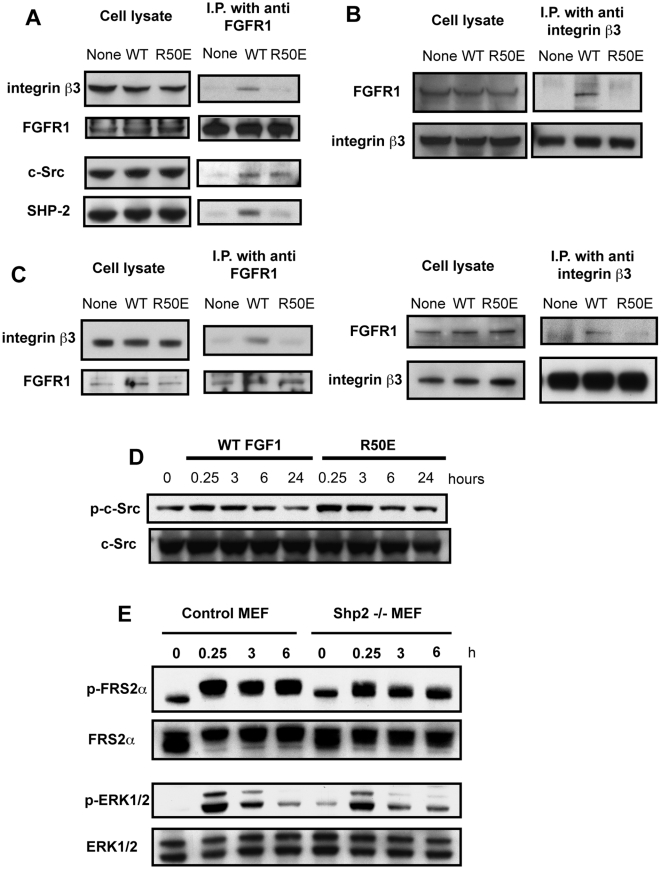
Figure 2. WT FGF1 induced FGFR1-FGF-αvβ3 ternary complex formation, but R50E was defective in this function.
We immunoprecipitated the FGFR1-αvβ3 complex from cell lysates with anti-FGFR1 (A) or anti-β3 mAb (B), and analyzed the immuno-purified materials by Western blot analysis. A. WT FGF1 induced co-immunoprecipitation of integrin β3 and SHP-1 with FGFR1 using anti-FGFR1, but R50E was defective in this function. B. WT FGF1 induced co-immunoprecipitation of FGFR1 with integrin β3 using anti-integrin β3, but R50E was defective in this function. We stimulated serum-starved NIH 3T3 cells with 5 ng/ml WT FGF1 or R50E for 1 h in the presence of 5 µg/ml heparin. C. Co-precipitation of integrin β3 and FGFR1 upon FGF1 stimulation in HUVEC. Serum-starved HUVEC were stimulated by 5 ng/ml WT FGF1 or R50E with 5 µg/ml heparin for 1 h. Cell lysates were immunoprecipitated with anti-FGFR1 or anti-β3 monoclonal antibody, and the immunoprecipitates were analyzed by Western blotting. D. WT FGF and R50E similarly activated c-Src. We stimulated serum starved NIH 3T3 cells with WT FGF1 or R50E, and cell lysates were analyzed by Western blotting using antibodies specific to phospho-c-Src or c-Src. E. Time-course of embryonic fibroblasts from SHP-2 (−/−) or control mice. Serum starved cells were treated with WT FGF1 or R50E (5 ng/ml) for the time indicated and cell lysates were analyzed by Western blotting.