Abstract
Mutations in genes coding for cadherin 23 and protocadherin 15 cause deafness in both mice and humans. Here, we provide evidence that mutations at these two cadherin loci can interact to cause hearing loss in digenic heterozygotes of both species. Using a classical genetic approach, we generated mice that were heterozygous for both Cdh23 and Pcdh15 mutations on a uniform C57BL/6J background. Significant levels of hearing loss were detected in these mice when compared to age-matched single heterozygous animals or normal controls. Cytoarchitectural defects in the cochlea of digenic heterozygotes, including degeneration of the stereocilia and a base-apex loss of hair cells and spiral ganglion cells, were consistent with the observed age-related hearing loss of these mice beginning with the high frequencies. In humans, we also have obtained evidence for a digenic inheritance of a USH1 phenotype in three unrelated families with mutations in CDH23 and PCDH15. Altogether, our data indicate that CDH23 and PCDH15 play an essential long-term role in maintaining the normal organization of the stereocilia bundle.
INTRODUCTION
Mutations in cadherin 23 (CDH23 ) and protocadherin 15 (PCDH15 ) are the causes of Usher syndrome types 1D and 1F, respectively, as well as certain forms of non-syndromic deafness (1–5). Both USH1D and USH1F map to human chromosome 10q21–q22 within a 15 cM interval. The murine orthologs are located ~10 cM apart on chromosome 10 and mutant forms of these genes give rise to the homozygous waltzer (Cdh23 ) and Ames waltzer (Pcdh15 ) phenotypes, respectively. Both mutant homozygotes have similar phenotypes. They exhibit deafness, vestibular dysfunction and disorganized, splayed stereocilia (6–9). Interestingly, recent evidence demonstrates that a strain-specific Cdh23 dimorphism is likely responsible for the modifier of deaf waddler (mdfw) and age-related hearing loss locus (ahl) phenotypes (10,11). Thus, Cdh23 may be an important gene that may be involved not only in certain forms of congenital deafness but also in age-related hearing loss and has the potential to genetically interact with other deafness genes to affect hearing. Both Cadherin 23 and protocadherin 15 belong to the cadherin super gene family that also includes classical cadherins, atypical cadherins, desmocollins, desmo-gleins and Flamingo cadherins (12). Cadherins support calcium-dependent cell–cell adhesion mainly through the formation of homophilic interaction (13). Such cell contacts are essential for compaction and cellular rearrangement during development and in adult (14). Cadherin 23 and protocadherin 15 share with the atypical cadherins, or cadherin-like proteins, homology in the extracellular domains and presumably mediate similar functions involving cell–cell or cell–extracellular matrix interactions. Given the functional, histologic and genetic similarities of pathogenic mutations at the cadherin 23 and protocadherin 15 genes, we sought to determine whether digenic interaction could be demonstrated between the two cadherin genes.
RESULTS
Progressive hearing loss in double heterozygous Cdh23v-2J +/+ Pcdh15av-3J mice
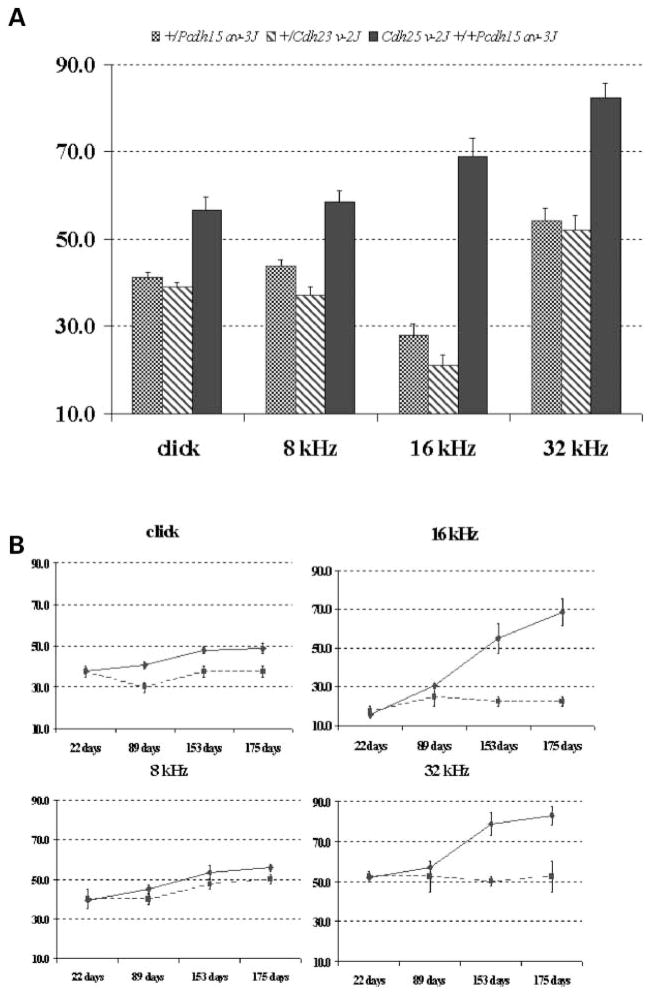
Both the Cdh23v-2J and the Pcdh15av-3J mutations occurred in colonies of C57BL/6J mice, and all of the genotype combinations tested were on this uniform strain background. Mice doubly heterozygous for these linked genes, Cdh23v-2J +/+ Pcdh15av-3J, and single heterozygotes, +/Cdh23v-2J and +/Pcdh15av-3J, were tested for auditory-evoked brainstem response (ABR) thresholds in response to broadband click and to 8, 16, and 32 kHz pure-tone stimuli, according to previously established criteria (15). As shown in Figure 1A, by 6–7 months of age, the double heterozygous Cdh23v-2J +/+ Pcdh15av-3J mice had significantly elevated ABR thresholds compared with single heterozygous +/Cdh23v-2J or +/Pcdh15av-3J mice. Of the 33 double heterozygotes tested at this age, 30 exhibited thresholds exceeding the normal range for mice according to the criteria (15), whereas none of the five +/Cdh23v-2J and only one of the 12 +/Pcdh15av-3J single heterozygotes tested exhibited above normal thresholds. The observed average threshold shifts for double heterozygotes ranged from 15 to 40 dB sound pressure level (SPL) above those of single heterozygotes, depending on the auditory stimulus, and were greatest at the highest frequencies tested (16 and 32 kHz).
Figure 1.
Elevated ABR thresholds and progression of hearing loss in double heterozygous mice. Average ABR thresholds for broadband clicks and 8, 16 and 32 kHz pure-tone stimuli are shown for 12 +/Pcdh15av-3J single heterozygotes (cross-hatched bars), five +/Cdh23v-2J single heterozygotes (diagonally marked bars) and 33 Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mice (black bars) tested at 6–7 months of age. ABR threshold values below 55 (click), 50 (8 kHz), 35 (16 kHz) and 60 (32 kHz) dB SPL are considered within the normal range for mice (15). The maximum amplitude presented for each test stimulus was 100 dB. Standard errors are indicated for each threshold mean (A). For each test stimulus, the average ABR thresholds of two +/Pcdh15av-3J single heterozygous controls (squares connected by dashed line) and seven Cdh23v-2J +/+ Pcdh15av-3J double heterozygotes (diamonds connected by solid line) are plotted for successive tests performed at 22, 89, 153 and 175 days of age. Standard errors are indicated for each threshold mean (B).
To evaluate hearing loss progression, seven double heterozygotes and two +/Pcdh15av-3J single heterozygotes were repeatedly tested for ABR thresholds at 22, 89, 153 and 175 days of age (Fig. 1B). At 22 days of age, there were no differences in ABR thresholds between single and double heterozygotes for any of the test stimuli, but by 153 days of age, the double heterozygotes had significantly elevated thresholds, especially for the 16 and 32 kHz stimuli (~+30 dB). Thus, double heterozygous mice exhibit a progressive and preferential loss of high-frequency hearing with onset at about five months of age. These findings demonstrate that an interaction between Cdh23v-2J and Pcdh15av-3J results in a progressive hearing loss in mice that are heterozygous for pathologic mutations at both loci.
Stereocilia of hair cells are degenerated in Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mice
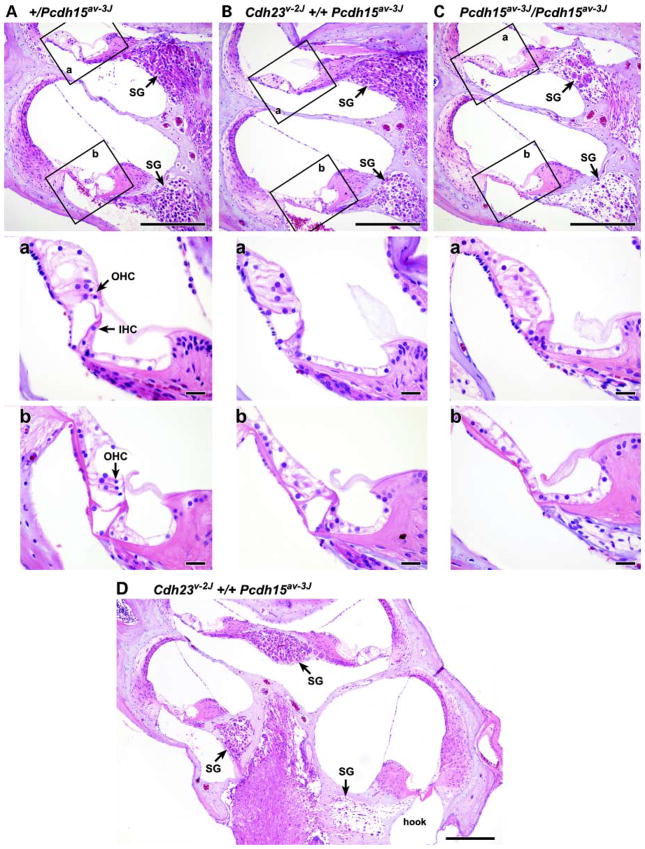
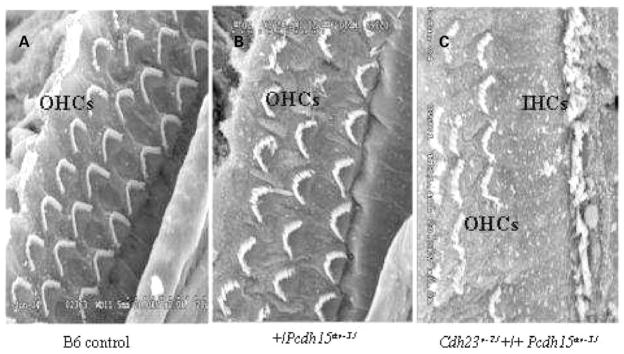
We compared the histological appearance of the organs of Corti of the single (+/Cdh23v-2J and +/Pcdh15av-3J) and double heterozygotes (Cdh23v-2J +/+ Pcdh15av-3J) with those of a single homozygous mutant mouse (Pcdh15av-3J/Pcdh15av-3J) by light microscopy (LM) and by scanning electron microscopy (SEM). Examinations of cross-sections through three baso-apical regions of the cochlea of double heterozygous mice at 6 months of age revealed mid-basal (basal turn of mid-modiolar section) and extreme basal (the basal hook) loss of hair cells. Cochleae of Cdh23v-2J +/+ Pcdh15av-3J double heterozygotes also exhibited loss of spiral ganglion cells (SGC) that was apparent in the mid-basal turn and more pronounced in the extreme base (Fig. 2B and D). SGC loss in these regions was confirmed by SGC density measurements performed as previously described (11) (Table 1). Cochlear degeneration in double heterozygous mice seemed to be specific to the hair cells and appeared not to affect the supporting cells of the organ of Corti. All other cochlear structures, including Reissner’s membrane and stria vascularis, appeared normal in double heterozygous mice. Analysis of the organ of Corti by SEM of the double heterozygous mice showed degenerated outer hair-cell stereo-cilia in contrast to the well-organized pattern and rigid structure observed in single heterozygotes and wild-type littermates. Inner hair-cell stereocilia of the double heterozygous mice also exhibit a degenerated appearance, but to a lesser degree than that observed in the outer hair cells (Fig. 3A–C). There is no evidence of hair-cell degeneration in the mid-apex (apical turn of mid-modiolar section, but below the apex) of the organ of Corti of the double heterozygous mice at 6 months of age (Fig. 2B), also there was no difference in SGC density in double heterozygous mice when compared with the age-matched single heterozygous animals (Table 1). In the cochlea, hair cells display region specificity and frequency selectivity. Along the longitudinal axis of the organ of Corti, ciliary bundles of hair cells have a gradation in length and their electrical properties also vary. Thus, there is an apical-to-basal gradient of sensitivity toward low to high frequencies of sound (16,17). Given these region-specific properties, defects detected in hair cells of 6-month-old double heterozygous mice correlate with the observation that double heterozygotes exhibit high-frequency hearing loss at that age.
Figure 2.
Loss of hair cells and SGCs in cochlea of mutant mice. Cross-sections through three apico-basal regions of cochlea 6 months of age are shown: mid-apex (apical turn of mid-modilar section, but below the apex), mid-base (basal turn of mid-modiolar section) and the extreme base. Cochlea of a +/Pcdh15av-3J single heterozygote control (A), a Cdh23v-2J +/+ Pcdh15av-3J double heterozygote (B) and a Pcdh15av-3J/Pcdh15av-3J homozygote (C). The middle and the lower panels show magnified images corresponding to the boxed areas a (mid-apex region) and areas b (mid-base region) in the upper panels, respectively. Note the loss of hair cells (lower panels) and loss of neurons (upper panels) from both the double heterozygote and the single homozygote cochlea. SGC loss is more pronounced in the hook (extreme base) of Cdh23v-2J +/+ Pcdh15av-3J double heterozygote (D) at different magnification. SG, spiral ganglion; IHC, inner hair cell; OHC, outer hair cell. Scale bars, 200 μm for upper panels and 20 μm for middle and lower panels.
Table 1.
SGC densities measurements in cochlea from 6-month-old +/Pcdh15av-3J single heterozygotes controls, Cdh23v-2J +/+ Pcdh15av-3J double heterozygotes and Pcdh15av-3J/Pcdh15av-3J single homozygotes
| Cochlear turn | +/Pcdh15av-3J | Cdh23v-2J +/+ Pcdh15av-3J | Pcdh15av-3J/Pcdh15av-3J |
|---|---|---|---|
| Mid-apex | 39.2 ± 1.5 | 38.4 ± 1.1 | 13 ± 0.7* |
| Mid-base | 34 ± 1.6 | 24.2 ± 1.3* | 8.4 ± 1.1* |
| Basal hook | 20.2 ± 1.3 | 5.2 ± 1.9* | 3.6 ± 1.1* |
SGC measurements (cells per 10 000 μm2) were obtained from four cochleae from each genotype.
Significantly different (P < 0.001) compared with +/Pcdh15av-3J.
Figure 3.
Stereocilia defect and loss of sensory hair cells of the Cdh23v-2J +/+ Pcdh15av-3J double heterozygote. Scanning electron micrographs of hair cell stereocilia in cochlea of 7-month-old mice. Stereocilia on the three rows of OHCs in a cochlea of a wild-type littermate B6 +/+ mouse (A) and of a +/Pcdh15av-3J single heterozygous mouse (B), with a normal, highly organized pattern, when compared with stereocilia of a Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mutant mouse shown in (C). The single row of inner hair cells is seen in (C). Hair cells loss in the double heterozygous mutants underlies the disrupted appearance of the hair cell pattern. OHC, outer hair cell; IHC, inner hair cell. Scale bar, 5 μm.
Eye phenotype
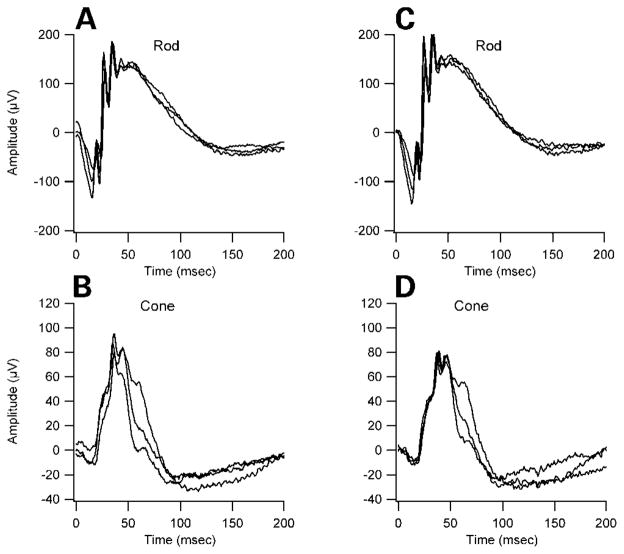
We examined the eyes of double heterozygous mice because mutations of the homologous genes in humans, CDH23 and PCDH15, cause progressive retinitis pigmentosa (RP), a characteristic of Usher syndrome. The eyes of the double heterozygous mice appear clinically normal on gross examination, having a normal fundus with thin retinal vessels at 7 months of age. The amplitude and implicit time of both the rod- and cone-mediated electroretinograms (ERGs) were comparable between double heterozygous Cdh23v-2J +/+ Pcdh15av-3J and single heterozygous littermates (Fig. 4). We found no evidence of anatomic defects in retinas of double heterozygous mice by histological examination (data not shown). Our findings are consistent with previous studies that have shown that retinal abnormalities associated with Usher syndrome are not present in homozygous mouse mutants defective for myosin VIIa (shaker-1), cadherin 23 (waltzer), protocadherin 15 (Ames walzer), usher 1g (jackson shaker) and usher 1c (deaf circler) (7,18–20). Although myosin VIIa, harmonin, cadherin 23 and protocadherin 15 are expressed in photoreceptor cells in both species (5,21), differences in the pattern of expression of the proteins in the retina in mice and humans may explain the absence of ocular defect in the mutant mice. Detailed characterization of the temporal and spatial expression pattern of human and mouse USH1 proteins will be needed to evaluate this possibility. It is also possible that the retinal disorder does not progress to detectable levels owing to the short life span of the mouse compared with humans. Finally, absence of retinal pathology in mouse models for USH1 may reflect interspecies differences in the functional requirements for these proteins or functional redundancy with other proteins in the retina or differences in other genetic, stochastic and environmental factors such as light exposure. Elucidation of the causes of this dissimilarity should shed light on the molecular or cellular pathways for potential interventions to prevent or delay RP in USH1.
Figure 4.
ERG waveforms from a 6-month-old Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mutant (A and B) and from an aged-matched +/Pcdh15av-3J single heterozygote control (C and D). The amplitude and implicit time of both the rod- and cone-mediated ERG are normal. Rod-dominated responses were recorded to short-wavelength (λmax = 470 nm; Wratten 47A filter) flashes of light over a 4.0 log unit range of intensities (0.3 log unit steps) up to the maximum allowable by the photic stimulator. Cone-dominated responses were obtained with white flashes (0.3 steps) on the rod-saturating background after 10 min of exposure to the background light to allow complete light adaptation. Signals were sample every 0.8 ms over a response window of 200 ms. For each stimulus condition, responses were computer-averaged with up to 50 records averaged for the weakest signals.
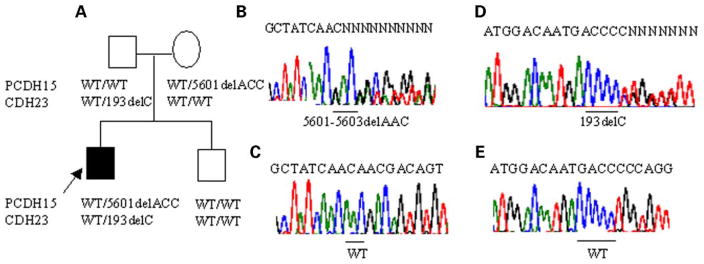
Evidence for interaction between CDH23 and PCDH15 in USH1 patients
Patients with USH1 are congenitally deaf, have vestibular dysfunction and generally develop RP in the first decade of life (22). In the present study, we carried out mutation screening for CDH23 and PCDH15 in 76 USH1 probands. In three simplex probands (4%), we found evidence for digenic inheritance of USH phenotype involving CDH23 and PCDH15. The patient in family 1121 from the UK (Fig. 5A) is a 36-year-old white male who presented with congenital profound deafness and had vestibular dysfunction. RP was diagnosed with ERG from childhood. The patient in family 239 from the US is a 16-year-old of European descent who had both congenital profound deafness and vestibular dysfunction. RP was diagnosed with ERG at age of 8 years and developed night blindness at the age of 13 years. A 43-year-old American female patient of African ancestry in family 1677 presented with congenital profound deafness. She developed balance problems at the age of 29 years. RP was diagnosed with ERG at age of 30 years and night blindness developed at the age of 40 years. Hearing impairment in these patients was congenital with no progression.
Figure 5.
Evidence for digenic inheritance of USH1 involving CDH23 and PCDH15. Pedigree and segregation of the mutations in CDH23 and PCDH15. The deaf proband is indicated by an arrow. CDH23/PCDH15 genotypes are given below the respective pedigree symbol (A). Direct sequence analysis showing the 5601delACC mutation (B) and wild-type (WT) allele (C) of PCDH15. Direct sequence analysis showing the 193delC mutation (D) and wild-type (WT) allele (E) of CDH23.
In family 1121 (Fig. 5A), the deaf proband was found to be heterozygous for 5601delAAC, a mutation in exon 33 of PCDH15 resulting in a deletion of a threonine residue at codon 1867, and for 193delC, a previously reported mutation of CDH23 in patients with USH1D (23). The PCDH15 cytoplasmic domain contains two proline-rich stretches that are conserved in evolution, which strongly imply that these domains are of functional significance for the proteins. Proline-rich sequences are among the most abundant motifs in eukaryotics cells and play a pivotal role in many signaling pathways (24). They mediate the assembly of molecular complexes by interacting with different domains contained in various proteins (25). The 5601delAAC mutation is located downstream of the proline-rich sequences of the PCDH15 protein and may, therefore, affect those functions. The 193delC mutation of CDH23 consists of a deletion of a single C in a CCCCC string in exon 3 located in the first extra-cellular repeat domain, which causes a frameshift at codon 65 and a stop codon 48 amino acids downstream. As the resulting truncated protein lacks ~96% of the predicted coding sequence, residual function is unlikely. Genotyping revealed that the mother was heterozygous for 5601delAAC of PCDH15, the father was heterozygous for 193delC of CDH23 and the normal hearing brother was homozygous for the wild-type alleles of both genes (Fig. 5A).
In family 239, the deaf proband was heterozygous for a 16delT mutation in exon 2 of PCDH15 and for a C→T transition at nucleotide 9565 of codon 3189 (R3189W) of CDH23 (data not shown). The normal hearing brother of the patient was found to be heterozygous only for the 16delT mutation in PCDH15. The PCDH15 16delT mutation causes a frame-shift leading to an altered amino sequence from codon 6, followed by a premature stop at codon11 in the predicted signal peptide sequence of PCDH15. The CDH23 cytoplasmic region contains two pdz-binding interfaces (PBI) domains, sequence motifs that are known to mediate protein–protein interactions: an internal domain with homology to a PBI in the adaptor protein Ril and a PBI at the C-terminal fitting the consensus for class I PBIs (26). The CDH23 R3189 codon located within the internal PBI and is conserved in evolution across species. The R3189W mutation in the cytoplasmic region of CDH23 protein might, therefore, affect the protein–protein interaction function of the protein rather than intercellular adhesion.
In family 1677 (data not shown), the deaf proband was heterozygous for 5601delAAC in PCDH15 and homozygous for an A→G transition at nucleotide position 3625 of CDH23 resulting in the substitution of a threonine for an alanine at codon 1209 (T1209A) of CDH23. This mutation has previously been identified in the homozygous state in patients with USH1D. It is located within a linker region between extracellular repeat domains 11 and 12 (23). Previous studies have shown that homozygous splicing variants, nonsense, frameshift mutations of CDH23 lead to typical USH1D (1,2,23,27), whereas homozygosity for missense mutations cause a form of non-syndromic deafness (DFNB12) or atypical USH (2,23). It is possible that the more severe USH phenotype found in the patient in family 1677 is due to a modifier effect of the PCDH15 5601delAAC mutation. The mutations identified in the present study are believed to be pathological, first, because of their location in functionally important and evolutionarily conserved domains and, secondly, because the changes were not observed in 100 unrelated control subjects.
DISCUSSION
In the present study, we show that mice doubly heterozygous for Cdh23v-2J and Pcdh15av-3J mutations exhibit deafness and abnormal stereocilia in outer and inner hair cells of the organ of Corti by 5 months of age, whereas single heterozygotes lack this pathology. Within the cochlea, Cdh23 and Pcdh15 are expressed in the inner and outer hair cells (6,7). In agreement with their expression pattern, we observed hair-cell specific defects in Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mice. Ultrastructural studies have revealed four types of stereocilia links including tip links, top links, shaft or lateral links and ankle links (28). They are thought to provide cohesiveness to the stereocilia bundle. Ankle links are present in mouse hair-cell stereocilia up to postnatal day 12 and then begin to disappear and are completely absent in adult mice (5). Cadherin 23 was first reported to be undetectable in stereo-cilia of mice older than postnatal day 30 (29), raising the possibility that it may be important for the development of stereocilia bundle and may have a role in the formation and maintenance of some transient links, such as ankle links, but not in the long-term maintenance of stereocilia bundle morphology. However, recent studies have indicated that CDH23 was still expressed in mature cochlear hair cells, even when hair cells have lost their kinocilium (30,31). It was suggested that its role in adult hair cells might be to form kinocilia links and tip links, which transmit force to mechanically gated ion channels. Protocadherin 15 is expressed in the stereo-cilia of developing mammalian hair cells and persists in adult hair cells along the length of stereocilia, indicating that it might be important for the long-term maintenance of lateral links between stereocilia (5). The prominent role of cadherins is in mediated cell-cell interactions (14). Thus, homophilic interaction of CDH23 and PCDH15 proteins might form links that interconnect stereocilia within a bundle. Both proteins could also engage in heterophilic interactions. Previous studies have suggested that interactions among the USH1-proteins myosin VIIa, harmonin, cadherin 23 and SANS may play a fundamental role in differentiation of the actin bundle in stereocilia (29,32). A failure of this process leads to stereocilia disorganization, as observed in mouse models (7,18–20), and is thought to be responsible for profound congenital deafness in patients with Usher syndrome (33). In the present studies, abnormal links between stereocilia could account for the stereocilia defect seen in double heterozygous mice. Loss of Cdh23 and Pcdh15 functions in heterozygous mice is progressive with aging, which indicates a crucial role for these proteins in the long-term maintenance of lateral connections between stereocilia.
Genetic fine mapping suggests that the mouse waltzer mutation (Cdh23v) is allelic with mdfw (10). We previously shown that Cdh23v might also be allelic to the ahl (11). Recent evidence demonstrates that a strain-specific dimorphism of Cdh23 likely underlies the mdfw and ahl phenotypes (10). Furthermore, it is known that in mouse, age-related hearing loss is subject to strain-specific effects. We have reported that the C57BL6/J background on which Cdh23v-2J and Pcdh15av-3J mutations are carried is susceptible to age-related hearing loss (11). Mice heterozygous for a presumed null allele of cdh23 (cdh23v) were shown by others to have low-and high-frequency hearing loss at 5–6 weeks of age, the high-frequency component of which worsens with increasing age (30). However, the hearing loss in these Cdh23v heterozygotes was dependent upon genetic background. In the present study, we clearly show that the hearing loss in Cdh23v-2J +/+ Pcdh15av-3J double heterozygous mice is due to a genetic interaction between Cdh23v-2J and Pcdh15av-3J rather than genetic background effects, because all mice tested were on a uniform C57BL/6J background. The observed genetic interaction might be a consequence of functional redundancy, as suggested by the following lines of evidence. First, both Cdh23- and Pcdh15-encoded proteins might be involved in lateral connections between stereocilia, because of their potential to participate in homophilic interactivity. Second, the phenotypic abnormalities of double heterozygotes are detected only in structures where both proteins are expressed. Finally, when defective, both proteins produce similar phenotypes.
The cytoplasmic domains of protocadherins, but not classical cadherins, are highly variable and contain various cytoplasmic sequences. It is, therefore, quite reasonable to assume that the protocadherins may have a variety of heterophilic interaction activities. The Cdh23v-2J mutation of the mice examined in the present study truncates 14 extracellular cadherin repeats, the putative transmembrane domain and the cytoplasmic tail. Although the truncated protein could possibly exist in secreted form and function as a dominant negative, the recessive inheritance of Cdh23v-2J argues in favor of a loss of function allele. The mutation in Pcdh15av-3J leads to the truncation of the cytoplasmic domain. As the mutant proteins in Cdh23v-2J and Pcdh15av-3J lack the C-terminal domains, mediation of the assembly via their tail domains is likely compromised. Our data together indicate that the genes encoding cadherin 23 and protocadherin 15 genetically interact to affect the long-term maintenance of the proper organization of the sterocilia bundle. However, we cannot exclude the possibility that they may have some role in their morphogenesis.
Here, we have provided evidence for hearing loss caused by an interaction of cadherin 23 and protocadherin 15 mutations in digenic heterozygotes in both mice and humans. However, the phenotypic outcome of the digenic heterozygotes is different in both species: patients with USH1 are congenitally deaf, whereas mice doubly heterozygous for Cdh23v-2J and Pcdh15av-3J have a progressive age-related hearing loss. It has previously been reported that the severity of phenotype in USH1D patients varies widely for the same mutation in the human population, even among members of the same family (2,23). This suggests that genetic background may be an important determinant in the extent of the pathology. Different genetic backgrounds could, therefore, be a possible explanation for the difference seen between humans and mice. Elucidation of the causes of this dissimilarity should advance our understanding of the underlying etiology of USH.
MATERIALS AND METHODS
Mice
Mice carrying the spontaneous Cdh23v-2J and Pcdh15av-3J mutations were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The strain background for both mutations is C57BL6/J. In Cdh23v-2J, a G→A transition at the first nucleotide of intron 32 of the cadherin 23 gene (Cdh23 ) alters the wild-type donor splice site (6). It introduces a premature stop codon. Pcdh15av-3J results from an insertion of a single A nucleotide after position 4521 in the coding region of the protocadherin 15 gene (Pcdh15 ) that leads to a frameshift and a premature stop codon. Both mutations are predicted to be null alleles. Matings between Pcdh15av-3J/Pcdh15av-3J mutant mice and +/+ mice produced obligate +/Pcdh15av-3J heterozygotes, and matings between Cdh23v-2J/Cdh23v-2J mutants and +/+ mice produced obligate +/Cdh23v-2J heterozygotes. Likewise, matings between Cdh23v-2J/Cdh23v-2J mutants and Pcdh15av-3J/Pcdh15av-3J mutants produced obligate Cdh23v-2J +/+ Pcdh15av-3J double heterozygotes. Additional mice with Cdh23v-2J genotypes can be obtained by mating +/Cdh23v-2J heterozygotes to Pcdh15av-3J/Pcdh15av-3J mutants. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Genotyping
Genomic DNA was amplified by polymerase chain reaction (PCR) and sequenced. Genotyping for the Pcdh15av-3J mutation was as previously described (7). PCR primers for the Cdh23v-2J mutation are V2-F: 5′-GCCAAAGCCCTCTTCAAGAT-3′ and V2-R: 5′-GGGAAAGCGTTTAGCTTGTT-3′, with the generation of a PCR fragment of 215 bp. Samples are sequenced on an automated sequencer (ABI-PRISM, model 3700).
Assessment of hearing by ABR
ABRs were conducted as previously described (15). Briefly, mice are anesthetized and their body temperature is kept at 37–38°C by placing them on a heating pad in the soundproof chamber during testing. Subdermal needle electrodes are inserted at the vertex (active) and ventrolaterally to the right ear (reference) and the left ear (ground). Specific auditory stimuli (broadband click and pure-tone pips of 8, 16 and 32 kHz) from high-frequency transducers are delivered binaurally through plastic tubes to the ears canals. Evoked brainstem responses are amplified and averaged and their wave patterns display on a computer screen. Auditory threshold are obtained for each stimulus by variant the SPL at 10 dB steps and finally at 5 dB step up and down to identify the lowest level at which an ABR pattern can be recognized.
Inner ear morphology
Inner ear morphology was examined by LM and SEM. Anesthetized mice were perfused through the left ventricle of the heart with phosphate-buffered saline followed by Bouin’s fixative. For microscopic analysis of cross-sections, inner ears from double heterozygote mutant and single heterozygote control mice were dissected, perfused with Bouin’s fixative, immersed in same for 24–48 h, decalcified with Cal-EX solution for 6 h and embedded in paraffin. Sections (7 μm) were cut, mounted on glass slides and counterstained in hematoxylin/eosin. For SEM analysis, inner ears from double heterozygous mutant mice and from single heterozygotes and wild-type controls were dissected and fixed in 2.5% glutaraldehyde, 0.25% tannic acid in 0.1 M phosphate buffer (pH 7.2) for 5 h at 4°C. The temporal bone surrounding the cochlea and the tectorial membrane were removed to expose the organ of Corti. An osmium tetroxide–thiocarbohydrazide procedure was used to stain prior to dehydrating and critical-point drying. Specimens were sputter coated with gold and examined at 15 kV under a Hitachi 3000N SEM.
Phenotypic evaluation of eyes
All mice had pupils dilated with 1% atropine ophthalmic drops and were evaluated by indirect ophthalmoscopy with a 78 diopter lens. Signs of retinal degeneration, such as vessel attenuation, alterations in the RPE, and presence or absence of retinal dots were noted. Methods for ERG and histological and electron microscopy of eyes were as previously described (20).
Mutation screening of CDH23 and PCDH15
The clinical and family history was obtained on each proband and complete physical examinations including audiological and ophthalmologic examinations were performed. Patients were identified as having Usher type I according to the criteria recommended by the Usher Syndrome Consortium (34). Approval for human subjects for this study was obtained from institutional review board at University of Miami. Informed consent was obtained for all participants. CDH23 is composed of 69 exons with a coding region of 10 062 bp. PCR amplification of CDH23 exons was performed using slightly modified versions of the primers previously reported (1). PCDH15 has 33 exons with a coding region of 5865 bp. Screening of the 33 exons of PCDH15 was performed using the previously described primers (3) with some modifications. PCR was performed in a 12.5 μl reaction with 40 ng of genomic DNA, 10 pmol of each primer, 200 μM dNTPs, 1.5 mM MgCl2 and 1 U of Taq DNA polymerase. The amplification conditions consist of 95°C for 5 min, then 30 cycles of 95°C for 1 min, 60°C for 1 min and 72°C for 1 min, with final extension for 5 min at 72°C. Samples were sequenced in both the forward and reverse directions on an automated sequencer (ABI-PRISM, model 3100).
Statistical analysis
All statistical analyses were performed with EXCEL.
Acknowledgments
We thank Fielding Hetjtmancik for providing patient’s DNA samples, Thomas Van de Water for helpful discussions of histology, Karen Steel and Walter Nance for their valuable comments on the manuscript, Beth Barr for histological service, Sandra Gray for providing the mouse breeders and Ronald E. Hurd for ERG testing. This work was supported by grants from National Institutes of Health DC05575 to X.Z.L., DC04301 to K.R.J. and DC005846 to Q.Y.Z.
Footnotes
References
- 1.Bolz H, von Brederlow B, Ramirez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del CSCM, Vila MC, Molina OP, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome typ.1D. Nat Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- 2.Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, Ramesh A, Schloss M, Srisailpathy CR, et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet. 2001;68:26–37. doi: 10.1086/316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Wilcox ER. Mutations of the protocadherin gene PCDH15 cause Usher syndrome typ.1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, et al. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet. 2001;10:1709–1718. doi: 10.1093/hmg/10.16.1709. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, Sieving P, Riazuddin S, Griffith AJ, Friedman TB, et al. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet. 2003;12:3215–3223. doi: 10.1093/hmg/ddg358. [DOI] [PubMed] [Google Scholar]
- 6.Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome typ.1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 7.Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet. 2001;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- 8.Holme RH, Steel KP. Stereocilia defects in waltzer (Cdh23), shaker1 (Myo7a) and double waltzer/shaker1 mutant mice. Hear Res. 2002;169:13–23. doi: 10.1016/s0378-5955(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 9.Raphael Y, Kobayashi KN, Dootz GA, Beyer LA, Dolan DF, Burmeister M. Severe vestibular and auditory impairment in three alleles of Ames waltzer (av ) mice. Hear Res. 2001;151:237–249. doi: 10.1016/s0378-5955(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 10.Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. Major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 12.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 13.Hynes RO. Specificity of cell adhesion in development: the cadherin superfamily. Curr Opin Genet Dev. 1992;2:621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 15.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 17.Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 19.Kikkawa Y, Shitara H, Wakana S, Kohara Y, Takada T, Okamoto M, Taya C, Kamiya K, Yoshikawa Y, Tokano H, et al. Mutations in a new scaffold protein Sans cause deafness in Jackson shaker mice. Hum Mol Genet. 2003;12:453–461. doi: 10.1093/hmg/ddg042. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, Zheng QY. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiners J, Reidel B, El-Amraoui A, Boeda B, Huber I, Petit C, Wolfrum U. Differential distribution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Invest Ophthalmol Vis Sci. 2003;44:5006–5015. doi: 10.1167/iovs.03-0483. [DOI] [PubMed] [Google Scholar]
- 22.Keats BJ, Corey DP. The Usher syndromes. Am J Med Genet. 1999;89:58–66. [PubMed] [Google Scholar]
- 23.Astuto LM, Bork JM, Weston MD, Askew JW, Fields RR, Orten DJ, Ohliger SJ, Riazuddin S, Morell RJ, Khan S, et al. CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet. 2002;71:262–275. doi: 10.1086/341558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini Ashburner M, Birney E, Boguski MS, Brody T, et al. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 26.Siemens J, Kazmierczak P, Reynolds A, Sticker M, Littlewood-Evans A, Muller U. The Usher syndrome proteins cadherin 23 and harmonin form a complex by means of PDZ-domain interactions. Proc Natl Acad Sci USA. 2002;99:14946–14951. doi: 10.1073/pnas.232579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Brederlow B, Bolz H, Janecke A, La O Cabrera A, Rudolph G, Lorenz B, Schwinger E, Gal A. Identification and in vitro expression of novel CDH23 mutations of patients with Usher syndrome type 1D. Hum Mutat. 2002;19:268–273. doi: 10.1002/humu.10049. [DOI] [PubMed] [Google Scholar]
- 28.Goodyear R, Richardson G. Distribution of the 275 kD hair cell antigen and cell surface specialisations on auditory and vestibular hair bundles in the chicken inner ear. J Comp Neurol. 1992;325:243–256. doi: 10.1002/cne.903250208. [DOI] [PubMed] [Google Scholar]
- 29.Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2004;5:66–79. doi: 10.1007/s10162-003-4021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 32.Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Laine S, Delmaghani S, Adato A, Nadifi S, Zina ZB, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet. 2003;12:463–471. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- 33.Petit C. Usher syndrome: from genetics to pathogenesis. Annu Rev Genomics Hum Genet. 2001;2:271–297. doi: 10.1146/annurev.genom.2.1.271. [DOI] [PubMed] [Google Scholar]
- 34.Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, Moller CG, Pelias MZ, Tranebjaerg L. Clinical diagnosis of the Usher syndromes. Usher syndrome consortium. Am J Med Genet. 1994;50:32–38. doi: 10.1002/ajmg.1320500107. [DOI] [PubMed] [Google Scholar]