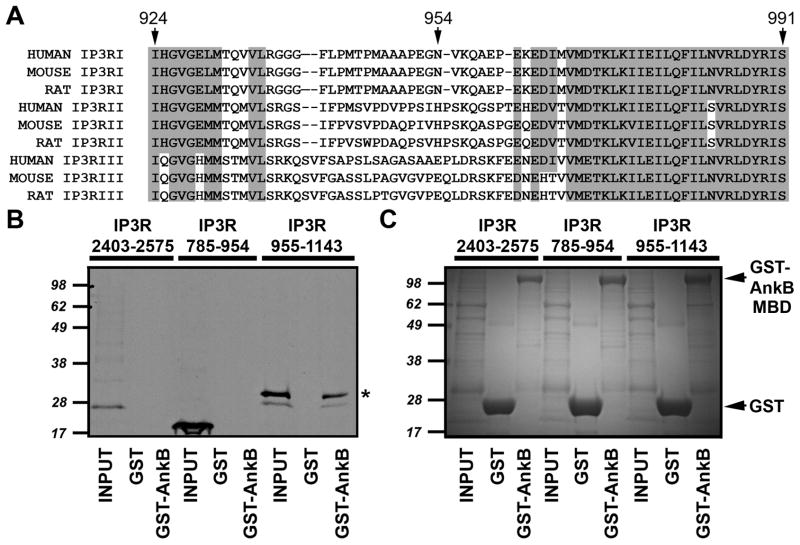
Figure 6. Ankyrin-B associates with InsP3 receptor residues 955–991.
A, Alignment of ankyrin-binding domain of InsP3 receptor. Sequences of human, mouse, and rat InsP3 receptor I, II, and III. Note high sequence conservation in N- and C-terminal regions of the domain. Residue numbers are based on rat InsP3 receptor amino acid sequence. B, InsP3 receptor ankyrin-binding domain (rat InsP3 receptor residues 924–991) was split into two constructs and in vitro translated in the presence of 35S-methionine. An InsP3 receptor C-terminal domain construct (residues 2403–2575) containing the previously predicted ankyrin-binding domain was also in vitro translated. Radiolabeled proteins were incubated with purified GST or GST-ankyrin-B membrane binding domain (GST-AnkB). Bound proteins were eluted and analyzed by SDS-PAGE and phosphorimaging. C, Gel used in binding studies was stained with Coomassie Blue to demonstrate protein purity and equal protein concentrations.