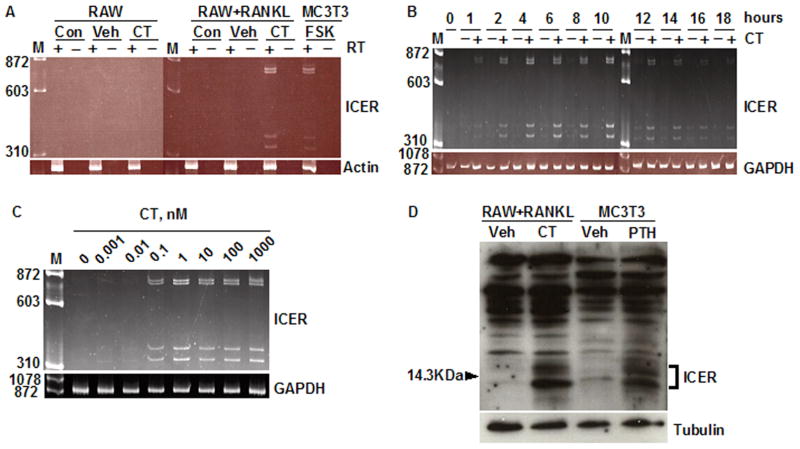
Fig. 1.
Calcitonin induces ICER expression in differentiated RAW264.7 cultures. (A) RAW264.7 cells (RAW) were cultured with or without RANKL for 4 days to induce osteoclast formation. At day 4, cultures were treated with vehicle (Veh) or 10 nM calcitonin (CT) for 2 h, or given no treatment (Con). RNA was extracted and treated with (+) or without (−) reverse transcriptase (RT) and PCR was performed using primers that detect all four of the ICER transcripts as described under Materials and Methods. A 2 h incubation of osteoblastic MC3T3-E1 cells with 10μM forskolin (FSK) served as a positive control for ICER induction [21]. M, molecular weight marker. β-Actin was used as a loading control. (B) RAW cells were treated with 30 ng/ml RANKL for 4 days to induce osteoclast formation and then treated with vehicle (−) or 10 nM calcitonin (CT) (+) for up to 18 h. RNA was extracted every 2 h and ICER transcripts assessed by RT-PCR. GAPDH was used as a loading control. (C) RAW264.7 cells were treated with 30 ng/ml RANKL for 4 days to induce osteoclast formation, and then treated with 0.01–1000 nM calcitonin (CT) for 2 h. ICER transcripts were assessed by RT-PCR. GAPDH was used as internal control. (D) RAW cells were treated with 30 ng/ml RANKL to induce osteoclast formation, and then treated with vehicle (Veh) or 10 nM calcitonin (CT) for 7 h. Nuclear extracts were subjected to western blotting and probed with an anti-CREM antibody. ICER proteins were seen as inducible, low-molecular-weight proteins bands around the 14.3 kDa marker. PTH treated MC3T3-E1 cells were used as positive control for ICER protein induction. Tubulin was used as a loading control. These experiments were performed at least twice with similar results.