Abstract
Soma® (carisoprodol) is an increasingly abused, centrally-acting muscle relaxant. Despite the prevalence of carisoprodol abuse, its mechanism of action remains unclear. Its sedative effects, which contribute to its therapeutic and recreational use, are generally attributed to the actions of its primary metabolite, meprobamate, at GABAA receptors (GABAAR). Meprobamate is a controlled substance at the federal level; ironically, carisoprodol is not currently classified as such. Using behavioral and molecular pharmacological approaches, we recently demonstrated carisoprodol, itself, is capable of modulating GABAAR function in a manner similar to central nervous system depressants. Its functional similarities with this highly addictive class of drugs may contribute to the abuse potential of carisoprodol. The site of action of carisoprodol has not been identified; based on our studies, interaction with benzodiazepine or barbiturate sites is unlikely. These recent findings, when coupled with numerous reports in the literature, support the contention that the non-controlled status of carisoprodol should be reevaluated.
Keywords: Carisoprodol, Discrimination, GABAA receptor, Meprobamate, Muscle relaxant, Substance abuse
Introduction
Carisoprodol was approved for clinical use as a muscle relaxant by the Food and Drug Administration in 1959 and was marketed under the trade name Soma® (Wallace Laboratories, Cranbury, NJ). It remains popular as a muscle relaxant, accounting for 21% of all skeletal muscle relaxant prescriptions in the United States in 2000 (1). According to IMS Health™, there were approximately 10 million prescriptions of carisoprodol issued in 2006 (2), supporting its continued use in clinical settings. The analgesic properties and muscle relaxant effects of carisoprodol are the bases for its use in the alleviation of lower back pain and in the short-term treatment of painful, acute musculoskeletal conditions. Like other muscle relaxants, carisoprodol is often prescribed as an adjunct to rest or physical therapy and is also available in preparations with other analgesics such as aspirin or codeine (Soma® Compound or Soma® Compound with codeine).
Carisoprodol as a drug of abuse
Although results from early studies in humans suggested carisoprodol did not have abuse- or dependence-producing potential (3), cases of carisoprodol abuse have been widely reported in the literature (4–9). Recent case studies have demonstrated the risk for tolerance, dependence, and withdrawal associated with carisoprodol use (10, 11). This drug often is used illicitly to combat opiate withdrawal or to enhance the sedative or euphoric effects of other CNS depressants (12–14). A report by Elder (9) ranked carisoprodol 54th among 234 drugs with abuse potential. As of 2000, the Drug Abuse Warning Network identified carisoprodol as the 20th most abused drug, ranking higher than oxycodone, methadone, and LSD (15).
Carisoprodol is listed as a drug/chemical of concern on the U.S. Department of Justice Drug Enforcement Agency Office of Diversion Control website (2), and its widespread abuse is becoming evident (16, 17). According to the Dallas DEA Field Division, carisoprodol is one of the six most commonly diverted drugs in its region (18). Along with benzodiazepines, Vicodin®, and OxyContin®, carisoprodol is one of the most commonly abused prescription drugs in Northern California (19). In Florida, the number of carisoprodol-/meprobamate (a metabolite of carisoprodol)-related deaths in 2005 exceeded those attributed to opioids, including heroin and fentanyl. Carisoprodol has also been directly and indirectly implicated in fatalities and suicide attempts (20–22). Although carisoprodol is not a controlled substance at the federal level, the incidence of its abuse is rising at such an alarming rate that it has prompted the states of Alabama, Arizona, Arkansas, Connecticut, Florida, Georgia, Hawaii, Kentucky, Massachusetts, Minnesota, Nevada, New Mexico, Oklahoma, Oregon, Virginia, and West Virginia to classify it as a schedule IV controlled substance (2, 23). An online search using the search string “Soma abuse” identifies the scope of the problem. Not only are numerous professional reports of tragic cases of Soma abuse returned, but links to many online pharmacies are retrieved. These pharmacy websites include statements such as “no prescription required” and “confidentiality assured”. In most cases, 250- or 350-mg tablets of generic Soma® can be acquired in large quantities from these sites at less than fifty cents per pill. Soma® abuse via internet pharmacies was also highlighted in the May 23, 2008 episode of the CNN series “Anderson Cooper 360” (24). Carisoprodol’s easy access and modest expense have no doubt contributed to the increasing abuse of this dangerous drug.
Reports of carisoprodol abuse have also been reported in India, Korea, Norway, and Sweden (4, 25–27). Recently, the Committee for Medicinal Products for Human Use (CHMP) concluded the abuse potential associated with carisoprodol outweighs its benefits as a therapeutic drug (28). Based on these findings, the European Medicines Agency recommended the suspension of the marketing authorization for all carisoprodol-containing products. Thus, abuse of carisoprodol has become an international problem.
Mechanism of abuse liability
There is a paucity of animal studies investigating the abuse liability of carisoprodol. Thus, mechanistic information underlying its abuse potential is sparse. Carisoprodol undergoes hepatic biotransformation by the cytochrome P450 enzyme 2C19 (CYP2C19). Hydroxylation and N-dealkylation produce three metabolic products—hydroxycarisoprodol, hydroxymeprobamate, and meprobamate (29, 30). In humans, the primary metabolite of carisoprodol is meprobamate (31). Meprobamate (Miltown®, Equanil®) is a sedative-hypnotic that was commonly used in the treatment of anxiety before its classification as a schedule IV controlled substance at the federal level. It thus has been generally accepted that both the therapeutic effects of carisoprodol and those that underlie its abuse potential are due to its conversion to meprobamate.
The GABAA receptor (GABAAR), the predominant inhibitory neurotransmitter receptor in the brain, has been implicated as a target of meprobamate. Meprobamate is barbiturate-like in its enhancement of benzodiazepine binding and inhibition of [35S]t-butylbicyclophosphorothionate binding at GABAARs (32, 33). Functionally, the actions of meprobamate in vivo have been likened to those of barbiturates (34). Furthermore, Rho et al. (35) demonstrated the barbiturate-like modulation of GABAAR function by meprobamate in vitro. These actions likely underlie the dangers associated with meprobamate toxicity and its potential for abuse.
In light of these findings, metabolism to meprobamate provides a reasonable explanation for the depressant, GABAergic effects attributed to carisoprodol. However, there is a distinction between carisoprodol toxicity and meprobamate toxicity, with the former being characterized by agitation and bizarre movement and the latter involving mainly CNS depression (36, 37). Moreover, these signs of toxicity are observed early in overdose, before carisoprodol is significantly converted to meprobamate (36). These findings suggest the actions of carisoprodol are dangerous in their own right and can be distinguished from those of meprobamate.
We have now discovered that carisoprodol itself has very prominent actions in the CNS (38). Initial studies were conducted at the whole-animal level to assess the likelihood that carisoprodol acts via the GABAergic system. Because carisoprodol produces perceptible CNS effects, we developed an animal model of the subjective effects of carisoprodol. In drug discrimination procedures, subjects learn to press one lever for food in the presence of drug and another lever in the presence of vehicle. These procedures have been useful in identifying pharmacological mechanisms for the subjective effects of drugs (39, 40) as well as for identifying potential abuse liability if novel compounds are shown to share subjective effects with known drugs of abuse (41, 42).
When we trained rats to discriminate carisoprodol from vehicle, the discriminative stimulus effects of carisoprodol were found to be comparable to those of the GABAergic ligands pentobarbital, chlordiazepoxide, and meprobamate, suggesting carisoprodol shares the substantial abuse liability of these compounds and that the stimulus effects of carisoprodol are mediated, at least in part, via GABAARs. Although both benzodiazepines and barbiturates substituted for carisoprodol, its effects were more consistent with those of barbiturates since its discriminative stimulus effects could be antagonized by a barbiturate antagonist, but not by a benzodiazepine antagonist. The question remained: is pentobarbital substituting for carisoprodol because of the barbiturate-like actions of meprobamate, or is carisoprodol mediating its own barbiturate-like effects? Some insight was gained in this regard based upon studies of the time course of motor depression elicited in response to carisoprodol. When administered orally to mice, carisoprodol produced motor depression of relatively short duration that was in accordance with its relatively short plasma half-life (43). Such an effect would not seem to be fully attributable to formation of meprobamate which has a half-life nearly eight-fold longer than carisoprodol. Nevertheless, meprobamate itself could also elicit motor depression, albeit with a longer apparent time course when compared with carisoprodol.
At the whole-animal level, it is difficult to distinguish the effects of the parent drug from its metabolite because metabolism begins virtually instantaneously. To circumvent the issues of metabolism, the effects of carisoprodol were examined using a simpler model system. Using human embryonic kidney 293 (HEK293) cells expressing human α1β2γ2 GABAARs, we demonstrated carisoprodol, like its metabolite, can allosterically modulate and directly gate the GABAAR. These effects can be described as barbiturate-like, and it is noteworthy that for both effects carisoprodol was more efficacious and potent than its metabolite, meprobamate. The barbiturate binding site remains elusive, preventing identification of a true barbiturate site antagonist. Although use of the barbiturate antagonist bemegride provided some information, we found its use somewhat limited for our in vitro studies. In the absence of such an important pharmacological tool, we assessed the potential role of the barbiturate site using a gain-of-function approach. Homomeric ρ1 GABA receptors, which are insensitive to barbiturates, gain sensitivity when tryptophan at position 328 is mutated to methionine (ρ1W328M, (44)). We generated this mutant and confirmed it did confer sensitivity to both the allosteric and direct gating effects of pentobarbital. However, carisoprodol did not allosterically potentiate or directly gate the ρ1W328M receptor. Thus, whereas our data, in general, demonstrate the actions of carisoprodol are “barbiturate-like”, these experiments indicate the binding and/or functional domains for the two ligands are not equivalent.
Both the γ-butyrolactones and neurosteroids also allosterically modulate and directly activate GABAARs (45). Whereas the stimulatory domains for lactones have not been identified, putative sites of action for neurosteroid modulations of GABAARs have been identified (46). These sites are located within the transmembrane domains of α and β subunits. Interestingly, the potentiating effects of neurosteroids are mediated by the α subunit whereas direct activation is dependent upon residues at the α/β interface (46). It will be interesting to determine whether these or novel sites are involved in mediating the effects of carisoprodol.
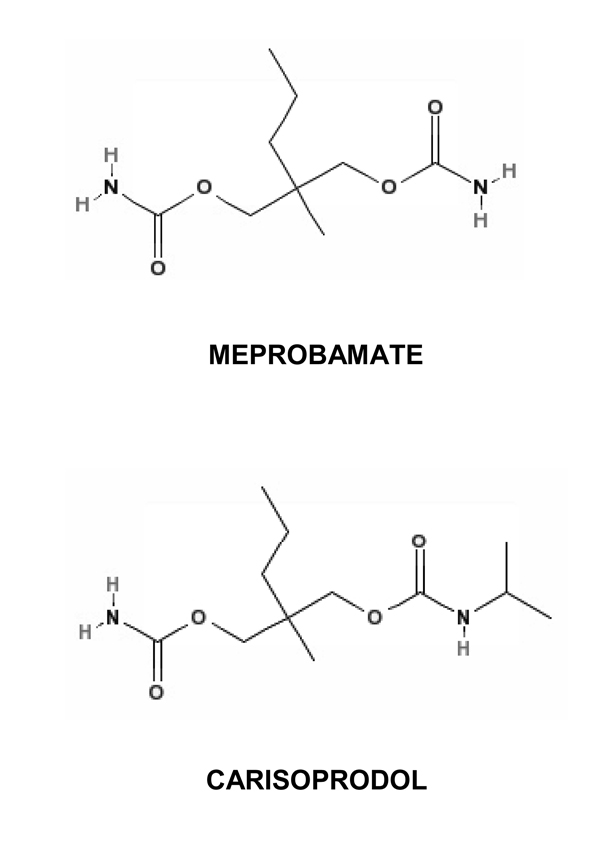
GABAAR subunit configuration varies regionally; thus, elucidating the subunit-dependence of carisoprodol may provide insight into its effects on certain areas of the brain that contribute to its therapeutic effects as well as its abuse potential. In preliminary studies (47), we have observed the actions of carisoprodol may be subunit-dependent, with sites of action likely on α and/or β subunits. Full understanding of the subunit-dependent effects of carisoprodol should also be informative in identifying regions of the receptor critical for the allosteric and direct gating actions of carisoprodol. In addition, the pharmacological profile of carisoprodol is not identical to that of meprobamate. These differences may be explained by distinct subunit-dependent effects of the drugs or possibly distinct sites of action. Given the structural similarities between carisoprodol and meprobamate (Figure 1), these reasons may not seem likely. However, felbamate, a propanediol dicarbamate structurally similar to meprobamate and carisoprodol, potentiates GABA-gated currents, but has no direct agonistic activity at these receptors (35). Thus, slight structural differences in this class of molecules can lead to drastic changes in drug-receptor interactions.
Figure 1. Chemical structures of carisoprodol and meprobamate.
Carisoprodol and meprobamate are propanediol dicarbamates. Carisoprodol was synthesized by replacing a hydrogen on a carbamyl nitrogen of meprobamate with an isopropyl group. Chemical structures were obtained from PubChem, an online resource made available through the United States National Library of Medicine (http://pubchem.ncbi.nlm.nih.gov.).
Carisoprodol abuse has been associated with dependence, tolerance, and withdrawal (10, 16). The CNS is constantly adapting to its environment; thus, it comes as no surprise that prolonged exposure to compounds elicits compensatory changes at the receptor level. Although we have not explored changes associated with chronic carisoprodol exposure, carisoprodol abuse is likely to elicit fundamental changes in the GABAergic system. Chronic exposure of the GABAAR to a number of allosteric modulators elicits changes in receptor subunit expression. In general, GABAARs are less sensitive to acute challenge following chronic exposure to a drug. This phenomenon may be due to uncoupling of allosteric sites (48), alterations in receptor turnover (49, 50), or desensitization. Whether expression of a subunit is upregulated or downregulated in response to chronic use varies with its location in the brain (51). It will be of interest to determine, along with developing knowledge of the subunit-dependent effects of carisoprodol, the extent to which chronic carisoprodol use may alter specific subunit expression and/or function. The shift towards configurations that are less sensitive to carisoprodol’s effects may contribute to tolerance because higher doses are needed to achieve the same effect. In addition, even subtle changes in inhibitory neurotransmission can have dire consequences. Such compensatory mechanisms associated with chronic activation of the GABAergic system are analogous to inhibitory dysregulation. Thus, abrupt removal of the drug is likely to precipitate withdrawal symptoms as the CNS attempts to restore normal inhibitory function. In addition, chronic opiate administration induces GABAARs in the ventral tegmental area to transition from inhibitory to excitatory signaling, acting as a switch for the dopaminergic reward pathway and contributing to opiate dependence (52). Although opiates are not GABAergic compounds, these findings demonstrate the involvement of GABAARs in the development of drug dependence.
Implications of carisoprodol findings
As noted, the abuse liability of carisoprodol is often attributed to its primary metabolite, meprobamate. Interestingly, meprobamate is a controlled substance at the federal level, but the parent drug, which appears to have similar effects (but with greater efficacy and potency), is not. The United States Food and Drug Administration uses an eight-factor analysis to determine whether a drug warrants legal scheduling (53). Factors include: 1) actual or relative abuse potential; 2) the historical and current pattern of abuse; 3) the scope, duration, and significance of abuse; 4) its risk to public health; 5) its potential for dependence liability; 6) whether the substance is a precursor of a controlled substance; 7) the state of current knowledge concerning the substance; and 8) scientific evidence of pharmacological effects. Whereas abuse potential, dependence, and potential health risks are well-documented, scientific evidence regarding carisoprodol’s pharmacological effects has been lacking in the literature. Our recent findings provide much needed information in this regard and suggest the nonscheduled status of carisoprodol should be reevaluated. Even if one were to presume the actions of carisoprodol are solely due to its conversion to meprobamate, continued non-scheduling of carisoprodol at the federal level, in light of meprobamate being scheduled, is illogical.
Moreover, while the number of reports regarding carisoprodol abuse continues to increase, there has been little progress in the treatment of carisoprodol dependence and withdrawal. At present, treatment consists of brief courses with benzodiazepines or phenobarbital to combat anxiety and insomnia. Furthermore, treatment of carisoprodol overdose is complicated as it is often characterized by agitation and seizures, and the administration of anticonvulsants and sedatives exacerbates CNS depression, leaving supportive therapy as a preferred course of action. Identification of the mechanism and site of action of carisoprodol may lead to more effective treatment of carisoprodol dependence and withdrawal and may provide information useful for the development of novel agents with reduced abuse potential.
Table 1.
Comparison of carisoprodol-, barbiturate-, and benzodiazepine-mediated modulation of GABAA receptors
| Carisoprodol | Barbiturates | Benzodiazepines | |
|---|---|---|---|
| In vivo | |||
| *Depression of locomotor activity | + | + | + |
|
*Substitution for the discriminative stimulus effects of carisoprodol |
+ | + | + |
| Antagonism by bemegride | + | + | −(54) |
| Antagonism by flumazenil | − | − | + (55) |
| In vitro | |||
|
*Potentiation of GABA-gated currents |
+ | + | + |
| • Antagonism by flumazenil | − | − | + |
| *Direct activation of GABAARs | + | + | − |
| • Antagonism by bemegride | + | + | ND |
| *Inhibition at high concentrations | + | + | − |
| *Elicits rebound currents | + | + | − |
| Regulation of GABAAR function in the absence of the γ2 subunit |
+ | + | − |
| Regulation of ρ1W328M receptor function |
− | + | ND |
an effect that was observed for meprobamate with results not significantly different from those of carisoprodol. Whereas meprobamate did not elicit inhibition nor rebound currents in our studies, these effects were reported in studies using higher concentrations of the drug (35).
+, −, and ND denote presence of effect, absence of effect, or not determined, respectively. Studies were conducted in parent labs, except where references are provided.
Acknowledgements
This work was supported in part by the National Institute on Drug Abuse (grant DA022370 and contract DA-2-8822, through the Addiction Treatment Discovery Program). LAG was supported by National Institute on Aging training grant T32-AG020494.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Luo X, Pietrobon R, Curtis LH, et al. Prescription of nonsteroidal anti-inflammatory drugs and muscle relaxants for back pain in the United States. Spine. 2004;29:E531–E537. doi: 10.1097/01.brs.0000146453.76528.7c. [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Justice Drug Enforcement Administration Office of Diversion Control. [cited 2009 Aug 12];Drugs and chemicals of concern--carisoprodol [internet] 2009 June; Available from: http://www.deadiversion.usdoj.gov/drugs_concern/carisoprodol.htm.
- 3.Eddy NB, Friebel H, Hahn KJ, et al. Codeine and its alternates for pain and cough relief. 2. Alternates for pain relief. Bull World Health Organ. 1969;40:1–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Sikdar S, Basu D, Malhotra AK, et al. Carisoprodol abuse: a report from India. Acta Psychiatr Scand. 1993;88:302–303. doi: 10.1111/j.1600-0447.1993.tb03462.x. [DOI] [PubMed] [Google Scholar]
- 5.Rust GS, Hatch R, Gums JG. Carisoprodol as a drug of abuse. Arch Fam Med. 1993;2:429–432. doi: 10.1001/archfami.2.4.429. [DOI] [PubMed] [Google Scholar]
- 6.Reeves RR, Pinkofsky HB, Carter OS. Carisoprodol: a drug of continuing abuse. J Am Osteopath Assoc. 1997;97:723–724. doi: 10.7556/jaoa.1997.97.12.723. [DOI] [PubMed] [Google Scholar]
- 7.Littrell RA, Hayes LR, Stillner V. Carisoprodol (Soma): a new and cautious perspective on an old agent. South Med J. 1993;86:753–756. doi: 10.1097/00007611-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Special report. Cady R. Skeletal muscle relaxants: A new rationale for choice. Prim Care Spec Ed. 2003;7:1–14. [Google Scholar]
- 9.Elder NC. Abuse of skeletal muscle relaxants. Am Fam Physician. 1991;44:1223–1226. [PubMed] [Google Scholar]
- 10.Heacock C, Bauer MS. Tolerance and dependence risk with the use of carisoprodol. Am Fam Physician. 2004;69:1622–1623. [PubMed] [Google Scholar]
- 11.Reeves RR, Algood TL, Wise PM. Skeletal muscle relaxants and associated medications for nonspecific acute back pain. P&T. 2005;30:518–524. [Google Scholar]
- 12.Chop WM., Jr Should carisoprodol be a controlled substance? Arch Fam Med. 1993;2:911. doi: 10.1001/archfami.2.9.911. [DOI] [PubMed] [Google Scholar]
- 13.Reeves RR, Carter OS, Pinkofsky HB. Use of carisoprodol by substance abusers to modify the effects of illicit drugs. South Med J. 1999;92:441. doi: 10.1097/00007611-199904000-00032. [DOI] [PubMed] [Google Scholar]
- 14.Reeves RR, Liberto V. Abuse of combinations of carisoprodol and tramadol. South Med J. 2001;94:512–514. [PubMed] [Google Scholar]
- 15.Drug Abuse Warning Network. [cited 2009 Aug 12];The DAWN report: narcotic analgesics. 2002 update [internet]. Available from: http://dawninfo.samhsa.gov/old_dawn/pubs_94_02/shortreports/files/DAWN_tdr_na2002.pdf.
- 16.Schwilke EW, Sampaio dos Santos MI, Logan BK. Changing patterns of drug and alcohol use in fatally injured drivers in Washington State. J Forensic Sci. 2006;51:1191–1198. doi: 10.1111/j.1556-4029.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 17.Reeves RR, Hammer JS, Pendarvis RO. Is the frequency of carisoprodol withdrawal syndrome increasing? Pharmacotherapy. 2007;27:1462–1466. doi: 10.1592/phco.27.10.1462. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JC. Sustance abuse trends in Texas. 2008. Jun, [cited 2009 12 Aug]. [internet]. Available from: http://www.utexas.edu/research/cswr/gcattc/documents/June2008.pdf. [Google Scholar]
- 19.United States Drug Enforcement Administration. DEA briefs & background, drugs and drug abuse, state factsheets, California [internet] [cited 2009 12 Aug];2008 March; Available from: http://www.usdoj.gov/dea/pubs/state_factsheets/california.html. [Google Scholar]
- 20.Robertson MD, Marinetti LJ. Carisoprodol--effects on human performance and behavior. Forensic Sci Rev. 2003:1–9. [PubMed] [Google Scholar]
- 21.Bailey DN, Briggs JR. Carisoprodol: an unrecognized drug of abuse. Am J Clin Pathol. 2002;117:396–400. doi: 10.1309/4KTM-CY6N-572P-7ERD. [DOI] [PubMed] [Google Scholar]
- 22.Akins BE, Miranda E, Lacy JM, et al. A multi-drug intoxication fatality involving Xyrem (GHB) J Forensic Sci. 2009;54:495–496. doi: 10.1111/j.1556-4029.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 23.Reeves RR, Burke RS. Is it time for carisoprodol to become a controlled substance at the federal level? South Med J. 2008;101:127–128. doi: 10.1097/SMJ.0b013e3181612062. [DOI] [PubMed] [Google Scholar]
- 24.Cable News Network. Keeping them honest. Anderson Cooper 360 [Google Scholar]
- 25.Jonsson A, Holmgren P, Ahlner J. Fatal intoxications in a Swedish forensic autopsy material during 1992–2002. Forensic Sci Int. 2004;143:53–59. doi: 10.1016/j.forsciint.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Chung H, Park M, Hahn E, et al. Recent trends of drug abuse and drug-associated deaths in Korea. Ann N Y Acad Sci. 2004;1025:458–464. doi: 10.1196/annals.1316.056. [DOI] [PubMed] [Google Scholar]
- 27.Bramness JG, Furu K, Engeland A, et al. Carisoprodol use and abuse in Norway: a pharmacoepidemiological study. Br J Clin Pharmacol. 2007;64:210–218. doi: 10.1111/j.1365-2125.2007.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. [cited 2009 Aug 12];WHO pharmaceuticals newsletter, No. 5 [internet] 2007 Available from: http://www.who.int/medicines/publications/newsletter/PN_No6_2007.pdf.
- 29.Douglas JF, Ludwig BJ, Schlosser A. The metabolic fate of carisoprodol in the dog. J Pharmacol Exp Ther. 1962;138:21–27. [Google Scholar]
- 30.Dalen P, Alvan G, Wakelkamp M, et al. Formation of meprobamate from carisoprodol is catalysed by CYP2C19. Pharmacogenetics. 1996;6:387–394. doi: 10.1097/00008571-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Olsen H, Koppang E, Alvan G, et al. Carisoprodol elimination in humans. Ther Drug Monit. 1994;16:337–340. doi: 10.1097/00007691-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Koe BK, Minor KW, Kondratas E, et al. Enhancement of benzodiazepine binding by methaqualone and related quinazolines. Drug Dev Res. 1986;7:255–268. [Google Scholar]
- 33.Squires RF, Casida JE, Richardson M, et al. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- 34.Roache JD, Griffiths RR. Lorazepam and meprobamate dose effects in humans: behavioral effects and abuse liability. J Pharmacol Exp Ther. 1987;243:978–988. [PubMed] [Google Scholar]
- 35.Rho JM, Donevan SD, Rogawski MA. Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate. J Pharmacol Exp Ther. 1997;280:1383–1391. [PubMed] [Google Scholar]
- 36.Roth BA, Vinson DR, Kim S. Carisoprodol-induced myoclonic encephalopathy. J Toxicol Clin Toxicol. 1998;36:609–612. doi: 10.3109/15563659809028058. [DOI] [PubMed] [Google Scholar]
- 37.Ellenhorn MJ, Barceloux D. Medical toxicology: diagnosis and treatment of human poisoning. New York: Elsevier Science Publishing; 1988. [Google Scholar]
- 38.Gonzalez LA, Gatch MB, Taylor CM, et al. Carisoprodol-mediated modulation of GABAA receptors: in vitro and in vivo studies. J Pharmacol Exp Ther. 2009;329:827–837. doi: 10.1124/jpet.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtzman SG, Locke KW. Neural mechanisms of drug stimuli: experimental approaches. Psychopharmacol Ser. 1988;4:138–153. [PubMed] [Google Scholar]
- 40.Woods JH, Bertalmio AJ, Young AM, et al. Receptor mechanisms of opioid drug discrimination. In: Colpaert FC, Blaster RL, editors. Transduction Mechanisms of Drug Stimuli. Berlin: Springer; 1988. pp. 95–106. [DOI] [PubMed] [Google Scholar]
- 41.Stolerman IP. Drug discrimination. In: Van Haaren F, editor. Methods in Behavioral Pharmacology. Amsterdam: Elsevier; 1993. pp. 217–243. [Google Scholar]
- 42.Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 43.van der Kleijn E. Kinetics of distribution and metabolism of ataractics of the meprobamate-group in mice. Arch Int Pharmacodyn Ther. 1969;178:457–480. [PubMed] [Google Scholar]
- 44.Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- 45.Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 46.Hosie AM, Wilkins ME, da Silva HM, et al. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez LA, Gatch MB, Forster MJ, et al. Carisoprodol acts at GABAA receptors in a subunit-dependent manner. Soc Neurosci Abst. 2008 531.9/D10. [Google Scholar]
- 48.Ito T, Suzuki T, Wellman SE, et al. Pharmacology of barbiturate tolerance/dependence: GABAA receptors and molecular aspects. Life Sci. 1996;59:169–195. doi: 10.1016/0024-3205(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 49.Pericic D, Strac DS, Jembrek MJ, et al. Prolonged exposure to gamma-aminobutyric acid up-regulates stably expressed recombinant alpha 1 beta 2 gamma 2s GABAA receptors. Eur J Pharmacol. 2003;482:117–125. doi: 10.1016/j.ejphar.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S, Kralic JE, O'Buckley TK, et al. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- 51.Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Laviolette SR, Gallegos RA, Henriksen SJ, et al. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- 53.Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70:S13–S40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 54.Schechter MD. Specific antagonism of the behavioral effects of chlordiazepoxide and pentobarbital in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:359–364. [PubMed] [Google Scholar]
- 55.Pugh SL, Boone MS, Emmett-Oglesby MW. Tolerance, cross-tolerance and withdrawal in rats made dependent on diazepam. J Pharmacol Exp Ther. 1992;262:751–758. [PubMed] [Google Scholar]