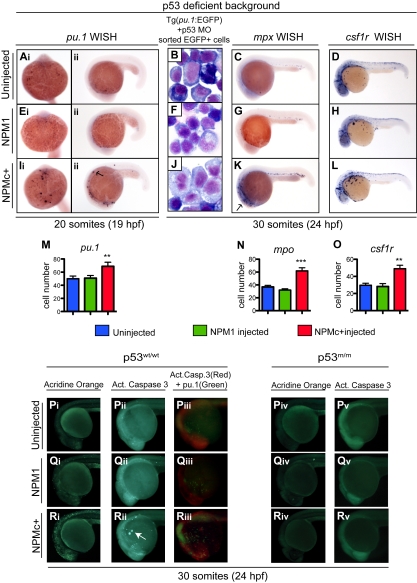
Figure 6.
NPMc+ expression causes an increase in the number of primitive mpx+ and csf1r+ cells in the absence of functional p53 and causes p53-dependent apoptotic cell death. (Ai-Aii, Ei-Eii, Ii-Iii, M) pu.1 WISH assays in homozygous p53 mutant embryos at 20 somites (19 hpf), uninjected (Ai-Aii), or injected with NPM1 10 pg (Ei-Eii) or NPMc+ 50 pg (Ii-Iii). Embryos are shown in ventral (Ai,Ei,Ii; anterior to the top) and lateral (Aii,Eii,Iii; anterior to the left, dorsal upwards) views. pu.1-expressing cells are indicated by dark purple spots and are increased in embryos injected with NPMc+, 50 pg (Ii-Iii). (M) pu.1+ cell number quantification shows a statistically significant increase upon NPMc+ expression (20 embryos counted per condition). (B,F,J) Giemsa stain of cystospins of EGFP+ cells sorted from Tg(pu.1:EGFP) embryos at 30 somites (24 hpf), injected with p53 MO 1.6 ng alone (B) or in combination with either NPM1 10 pg (F) or NPMc+ 50 pg (J). NPMc+ expression results in larger cells with more immature morphology (J). Images were acquired with a Zeiss Axio imager Z1 microscope using a Zeiss 63×/1.4 NA Apochromat Oil lens (Carl Zeiss) and Openlab software (Perkin Elmer). (C-D, G-H, K-L, N-O) mpx (C,G,K) and csf1r (D,H,L) WISH are shown in lateral views (anterior to the left, dorsal upwards) of homozygous p53 mutant embryos at 30 somites (24 hpf) uninjected (C-D) or injected with NPM1 10 pg (G-H) or NPMc+ 50 pg (K-L). Note that mpx and csf1r expression is markedly expanded upon NPMc+ expression and p53 loss (K-L). The number of mpx and csf1r cells is quantified in panels N and O, respectively (20 embryos counted per condition). Note that the csf1r probe also stains xanthophores in the dorsal trunk of the embryo. Error bars represent SEM. *Statistically significant differences between the NPMc+-injected and both NPM1-injected and uninjected embryos (**P < .005; ***P < .001; Student t test). In histograms, blue indicates uninjected control embryos; green, NPM1-injected embryos; and red, NPMc+-injected embryos. (P-R) Wild-type p53 embryos are shown either uninjected (Pi-Piii), or expressing NPM1 (Qi-Qiii) or NPMc+ (Ri-Riii). All embryos are at 30 somites (24 hpf) and shown in lateral views of the head and anterior trunk, anterior to the left and dorsal upwards. Acridine orange staining (green indicate dead or dying cells) of embryos uninjected (Pi), or injected with NPM1 10 pg (Qi) or NPMc+ 50 pg (Ri) mRNA. An increase in dying cells is observed upon NPMc+ expression (Ri). Activated caspase-3 immunostaining (green indicates cells in apoptosis) of embryos uninjected (Pii), or injected with NPM1 10 pg (Qii) or NPMc+ 50 pg (Rii) mRNA. Increased numbers of apoptotic cells are observed in NPMc+-injected embryos (Rii), forming aggregates on the yolk (arrow). Anti–activated caspase-3 and anti-GFP immunostaining in Tg(pu.1:EGFP) transgenic embryos (red spots indicate cells that express the cleaved form of caspase-3, and green spots indicate cells expressing EGFP under the control of the pu.1 promoter), uninjected (Piii), or injected with NPM1 10 pg (Qiii) or NPMc+ 50 pg (Riii) mRNA. Note in panel Riii the increase in activated caspase-3–expressing cells that surround and lie adjacent to EGFP+ cells, but do not colocalize with them. Homozygous mutant p53 embryos are shown uninjected (Piv-Pv), or expressing either NPM1 (Qiv-Qv) or NPMc+ (Riv-Rv). All embryos are at 30 somites (24 hpf) shown in lateral views of the head and anterior trunk, anterior to the left and dorsal upwards. Acridine orange staining of embryos uninjected (Piv) or injected with NPM1 10 pg (Qiv) or NPMc+ 50 pg mRNA (Riv) show no difference in the number of dead or dying cells. Anti–activated caspase-3 staining of embryos uninjected (Pv) or injected with either NPM1 10 pg (Qv) or NPMc+ 50 pg (Rv) mRNA show no difference in the number of apoptotic cells. Embryos were equilibrated in glycerol and visualized with a Nikon SMZ1500 zoom stereomicroscope (Nikon) using a 488 nm filter for the EGFP signal and 568 nm filter for the red signal. Images were acquired with NIS-Elements software (Nikon).