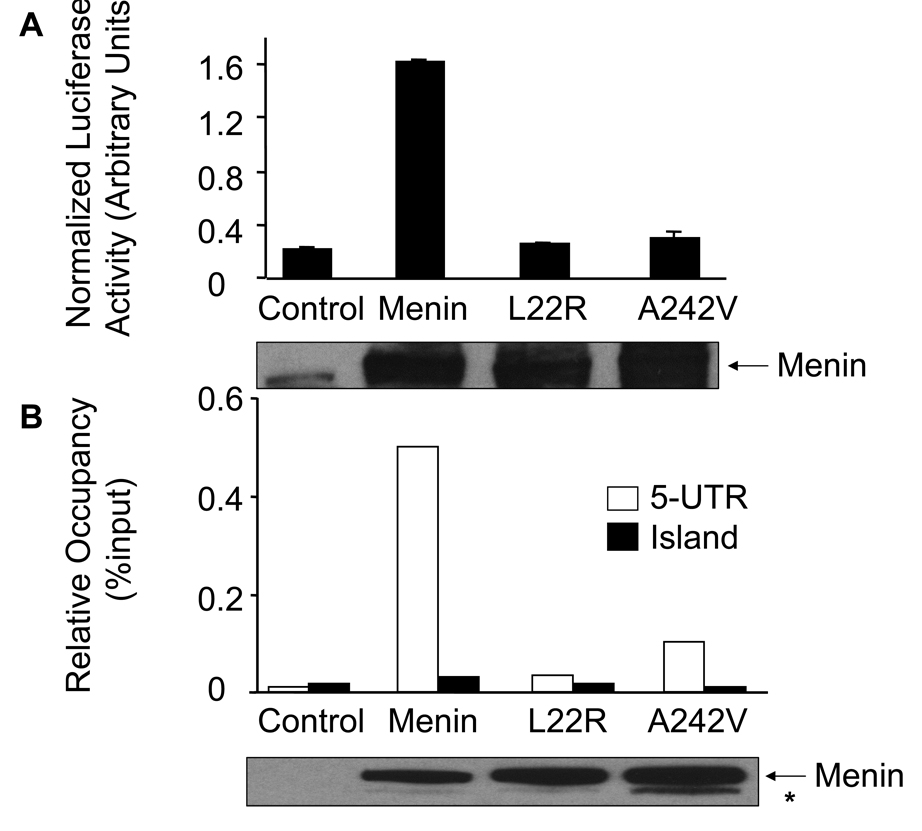
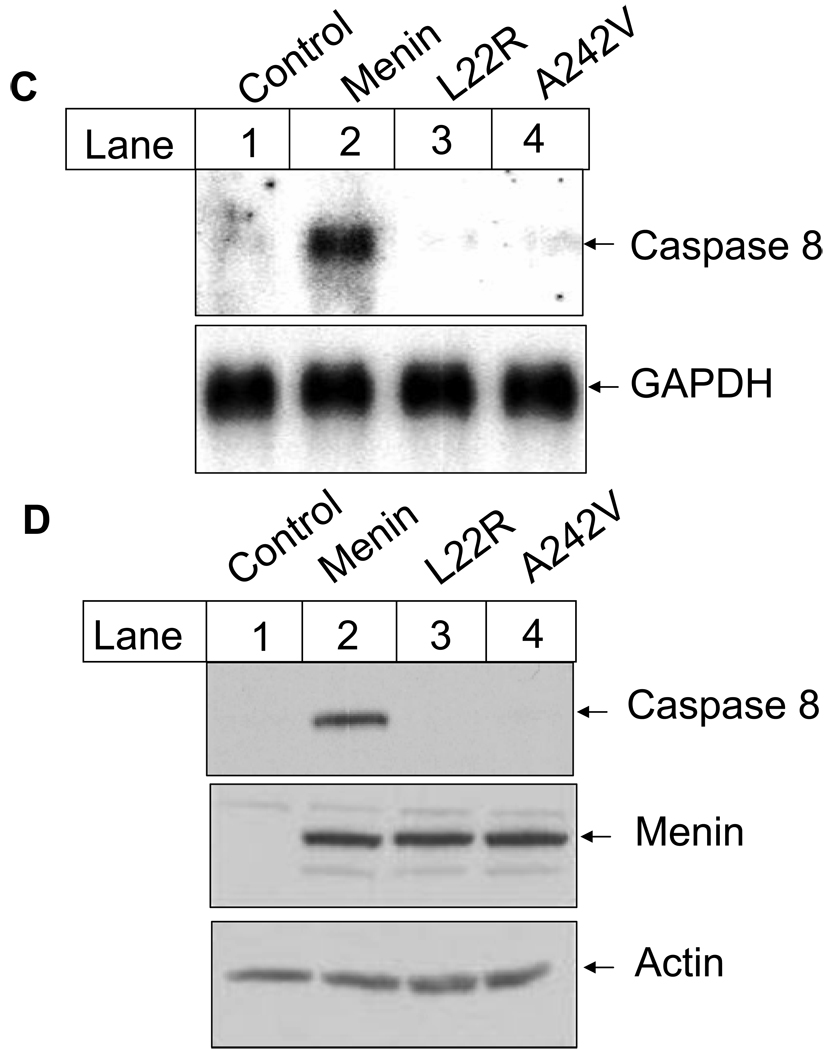
Fig. 5.
MEN1-related menin point mutants reduce their ability to induce expression of caspase 8 in vitro and in vivo. (A) Luciferase assays were performed as described in Fig. 3B and in EXPERIMENTAL PROCEDURES. These data were representative of three independent experiments. Expression of wild type and mutant menin was detected using Western blotting (bottom panel).
(B) MEN1-related point mutants of menin failed to bind the 5’-UTR of the caspase 8 locus. The various cells were used for ChIP assay as indicated in Fig. 4. The experiment was performed independently three times. Expression of wild type and mutant menin was detected using Western blotting (bottom panel). Asterisk (*) represents likely a proteolyzed menin product.
(C) Menin mutants fail to induce caspase 8 expression at the mRNA level. Cells described in Fig. 5B were used for Northern blotting with probes for murine caspase 8 and GAPDH. Total RNA was isolated from exponentially growing MEFs using cesium chloride ultracentrifuge method. RNA (20µg) was separated on agarose gel and transferred to Hybon N+ membrane. The membranes were further incubated with the labeled caspase 8 probe or the GAPDH probe, followed by autoradiography.
(D) Menin point mutants fail to restore caspase 8 expression at the protein level. Menin-null MEFs or MEFs expressing either wild type menin or its mutants were used. Caspase 8 and menin were detected using corresponding primary antibodies and secondary antibodies (top and middle panel), as described in EXPERIMENTAL PROCEDURES. To confirm the equal load of protein for caspase 8 and menin, the same amount of total protein (50µg) was also subjected to the detection with anti-actin antibody (bottom panel). It appears that the affinity of the anti-menin antibody to the wild type menin or the mutants was similar.