Figure 4. Oligomerization of Bcl-2 and Bax in liposomal membrane.
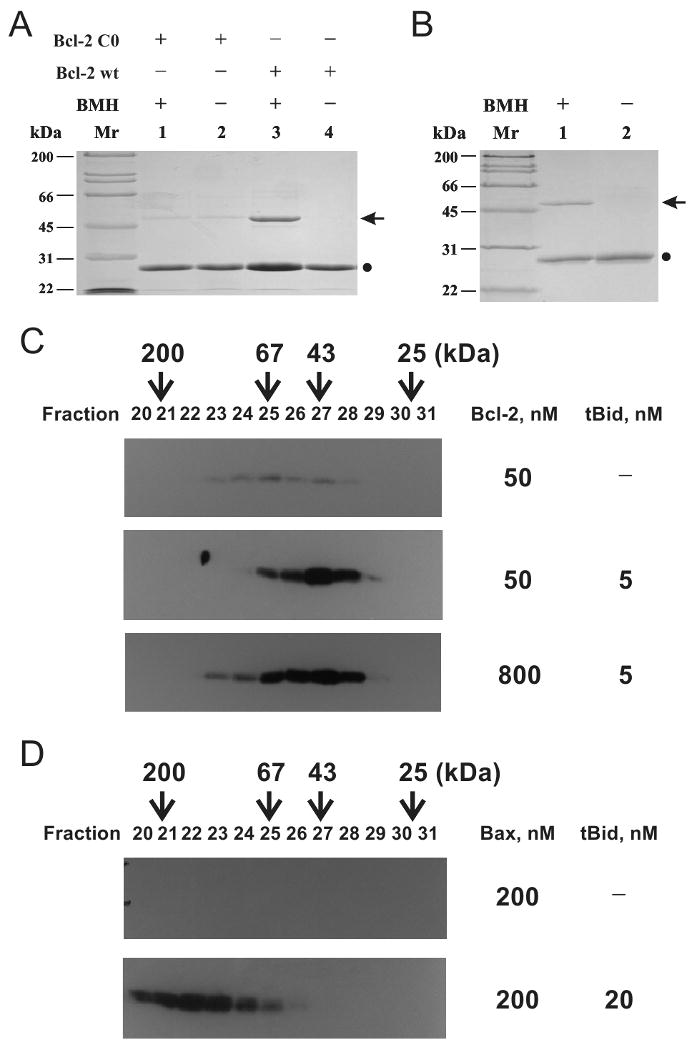
A, homo-association of His6-Bcl-2ΔTM in solution (pH 7.4) was detected by crosslinking with BMH. Data shown is a representative Coomassie-stained SDS-PAGE gel from three independent experiments. (●), Bcl-2 monomer; arrow, crosslinked Bcl-2 dimer. B, homo-association of His6-Bcl-2ΔTM in the liposomal membrane was monitored by BMH crosslinking after the protein was incubated with the liposome at pH 5.0, and the membrane-bound protein was purified using sucrose float-up centrifugation. Data shown is a representative from two independent experiments. C, oligomerization of 50 or 800 nM His6-Bcl-2ΔTM in the membrane was determined by gel filtration chromatography after incubating the protein at pH 7.4 with 12.5 μM Ni2+-liposome in the absence or presence of 5 nM tBid. The proteins in the eluted fractions were analyzed by SDS-PAGE and immunoblotting with a Bcl-2-specific antibody. The elution positions of protein standards are indicated on the top of the plot with Mr. D, oligomerization of 200 nM Bax in the membrane in the absence or presence of 20 nM tBid was determined similarly, except that a Bax-specific antibody was used in the immunoblotting.
