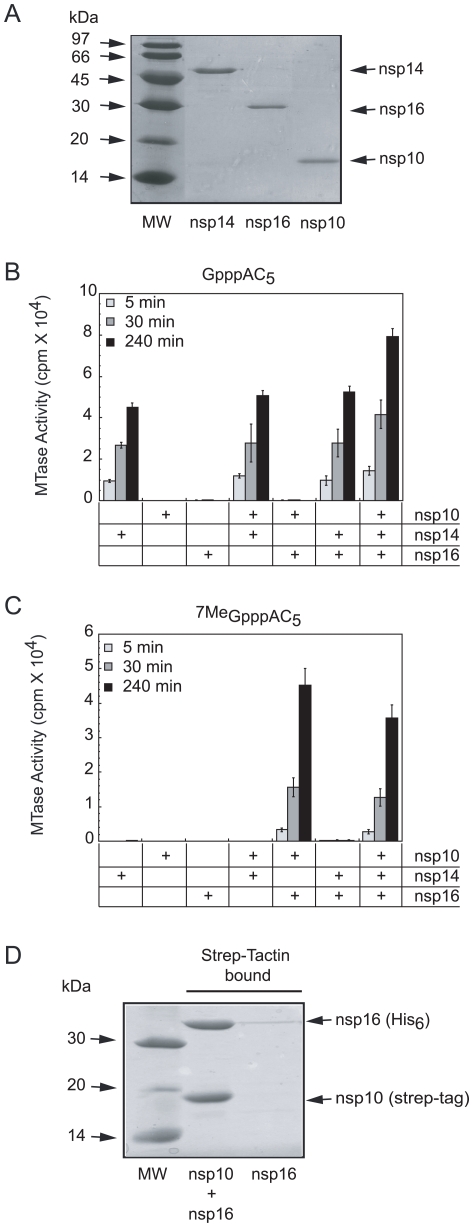
Figure 2. SARS-CoV proteins nsp14, nsp16 and nsp10 purification, AdoMet-dependent MTase activity on short capped RNA and complex formation of nsp16/nsp10.
Panel A: The SARS-CoV proteins nsp14, nsp16 and nsp10, purified by affinity and size exclusion chromatography (as described in Materials and Methods) were separated by SDS-PAGE (14%) and visualized by Coomassie blue staining. Lane 1 corresponds to the molecular size markers, lanes 2 to 4 to nsp14, nsp16, and nsp10, respectively. Panel B and C: AdoMet-dependent MTase assays performed on short capped RNA substrates. The different purified proteins (nsp10: 1200 nM, nsp14: 50 nM and nsp16: 200 nM) were incubated with GpppAC5 and 7MeGpppAC5 RNA oligonucleotides in presence of [3H]-AdoMet as described in Materials and Methods. The methyl transfer to the capped RNA substrate was determined after 5-, 30-, and 240-min incubation by using a filter-binding assay (see Materials and Methods). Panel D: SARS-CoV His6-nsp16 protein co-expressed with strep-tag-nsp10 and His6-nsp16 expressed alone were incubated with Strep-Tactin sepharose. Strep-Tactin-bound protein was eluted with D-desthiobiotin and analysed by SDS-PAGE and Coomassie blue staining. Lane 1 corresponds to the molecular size markers, lane 2 to strep-tag-nsp10 co-expressed with His6-nsp16 and lane 3 to His6-nsp16 alone.