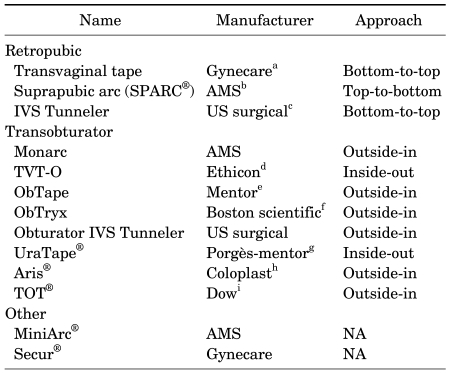
TABLE 1.
Commercial kits available for midurethral sling placement
NA: not applicable, TVT-O: transobturator vaginal tape, IVS: intravaginal slingplasty, All these kits use a polypropylene mesh sling. a: Gynecare® Inc., Menlo Park, CA. b: American Medical Systems Research Corp., Minnetonka, MN. c: US Surgical, Norwalk, CT. d: Ethicon Inc., Somerville, NJ. e: Mentor Corp., Santa Barbara CA. f: Boston Scientific Scimed Inc., Maple Grove, MN. g: Le Plessis, Robinson, France. h: Coloplast A/S, Copenhagen, Denmark, i: Dow Medics, Korea