Abstract
Live-attenuated influenza vaccine (LAIV) prevents significantly more cases of influenza in immune-competent children than the trivalent inactivated vaccine (TIV). We compared the T cell responses to LAIV or TIV in HIV-infected children. IFN-γ-ELISPOT for the three vaccine-contained influenza strains, two mismatched strains, and phytohemagglutinin (PHA), was performed at 0, 4, and 24 weeks postimmunization in 175 HIV-infected children randomly assigned to LAIV or TIV. The contribution of CD8 T cells to the influenza-specific response (CD8-ELISPOT) was evaluated by CD8-cell depletion. CD8 T cells accounted for ≥87% of the total influenza-ELISPOT. At baseline, total influenza-ELISPOT and CD8-ELISPOT values were similar or higher in TIV compared with LAIV recipients. Four and 24 weeks after TIV, total influenza-ELISPOT and CD8-ELISPOT results were significantly lower than baseline results (p ≤ 0.001). Responses to PHA also tended to decrease at 4 weeks after TIV (p = 0.06), but rebounded to baseline levels at 24 weeks. Four weeks after LAIV, total influenza-ELISPOT responses to vaccine-contained strains A H3N2 and B significantly decreased. Other ELISPOT values at 4 weeks and all values at 24 weeks were similar to the baseline values. At 4 and 24 weeks, TIV compared to LAIV administration resulted in a significantly greater decrease in influenza-specific ELISPOT values for vaccine-contained influenza A strains (p ≤ 0.02). Responses to PHA also tended to decrease more in TIV recipients (p = 0.07).
HIV-infected children immunized with TIV had significant and persistent decreases in ELISPOT responses to influenza. LAIV administration suppressed ELISPOT responses less. The clinical significance of these findings deserves further study.
Introduction
Yearly immunization of HIV-infected individuals with inactivated trivalent influenza vaccine (TIV) is recommended, although its effectiveness in these patients has not been established. Because antibody responses to TIV are not always adequate in this population,1,2 immunization of household contacts is strongly encouraged as a means of protecting HIV-infected individuals against influenza. A live attenuated influenza vaccine (LAIV) is licensed in the United States for immunization of healthy individuals 2 to 49 years of age. This vaccine has been well tolerated and immunogenic in previously immunized HIV-infected children and adults, but its efficacy in this population has not been established.3–5 LAIV is more effective than TIV in healthy children and confers protection against infection with mismatched strains of influenza.6,7
Serum antibody responses to LAIV, unlike those to TIV, do not correlate with protection against wild-type infection and the mechanism(s) of protection by LAIV is not completely understood.8,9 Cell-mediated immunity (CMI), particularly CD8-mediated cytotoxicity, plays an important role in protection against influenza in animal models.10–13 Less is known about the role of CMI in human influenza, but it may play an important protective role.8,14–17 A recent study associated ELISPOT responses to influenza vaccines of ≥100 spot-forming cells (SFC)/106 peripheral blood mononuclear cells (PBMCs) with protection against influenza infection in children immunized with LAIV,18 suggesting that CMI contributes to the protective effect of LAIV.
In HIV-infected individuals, vaccine-induced CMI may play a direct role in protection against infection and provide critically needed help to B cell antibody production. B cell numbers and function are compromised in HIV-infected hosts, leading to inadequate antibody responses to vaccines.19–21 In addition, T cell-dependent vaccines are more immunogenic in HIV-infected hosts than T-independent vaccines,22 underscoring the importance of using a T cell-inducing immunogen in these individuals. We recently showed that antibody responses to hepatitis A virus vaccine are higher in HIV-infected children who acquired hepatitis A virus-specific CMI after immunization compared with those who did not.23
The objectives of this study were to compare influenza-specific CMI responses to LAIV and TIV in HIV-infected children and to assess potential associations between the CMI response and protection against shedding of live attenuated vaccine viruses.
Materials and Methods
Study design (IMPAACT P1057)
HIV-infected children and adolescents aged 5 to 18 years were randomly assigned to receive LAIV (Arm A) or TIV (Arm B) in the autumn of 2004. Inclusion criteria were stable combination antiretroviral therapy for ≥16 weeks prior to immunization; plasma HIV RNA <60,000 copies/ml and CD4 ≥15% within 60 days of enrollment; and immunization with TIV at least once in the 2 years preceding the study. Potential subjects were excluded if they received immunomodulatory therapy within 60 days prior to enrollment, inactivated or live vaccines within 14 and 30 days, respectively, or if they met the safety exclusion criteria listed in the package inserts of either vaccine. In each study arm the vaccinees were stratified by the following nadir CD4% criteria: Group 1 <15%; Group 2 ≥15% but <25%; and Group 3 ≥25%.
Arm A (LAIV) received the frozen formulation of Influenza Virus Vaccine Live, Intranasal (FluMist; MedImmune) 0.5 ml (0.25 ml per nostril) and Arm B (TIV) received Influenza Viral Vaccine, Intramuscular (Fluzone; Aventis Pasteur, Inc.) 0.5 ml in the deltoid muscle region. Both vaccines were stored and administered according to the package insert. The strains represented in the vaccines were those recommended by the U.S. Public Health Service (USPHS) for the 2004/2005 season: A/New Caledonia/20/99 (H1N1); A/Wyoming/3/2003 (H3N2) (an A/Fujian/411/2002-like virus); and B/Jilin/20/2003 (LAIV) or B/Jiangsu/10/2003 (TIV) (both Yamagata lineage, B/Shanghai/361/2002-like viruses).
Study population for this analysis
The first 25–30 subjects in each Arm/Group combination were enrolled in the ELISPOT substudy. Blood was obtained at 0, 4, and 24 weeks after immunization.
Laboratory analyses
Interferon (IFN)-γ ELISPOT responses were assessed on fresh shipped PBMCs as previously described.24 PBMCs separated with Ficoll-Hypaque gradients were stimulated for 16–20 h in vitro with 10 TCID50/cell of attenuated monovalent influenza virus corresponding to the vaccine strains (A H1N1 New Caledonia, A H3N2 Wyoming, and B Jilin); with mismatched influenza strains (A H3N2 Sydney and B Yamanashi); and with medium and phytohemagglutinin (PHA) (5 μg/ml) controls. Spots were visualized using a CTL ELISPOT plate reader. Background (nonspecific) spots detected in the medium-containing wells were subtracted from the wells stimulated with influenza antigens. Results were reported as SFC/106 PBMCs.
To assess the contribution of CD8 T cells to the influenza-specific responses measured by ELISPOT, an aliquot of PBMCs was depleted of CD8 cells using magnetic beads coated with anti-CD8 monoclonal antibodies (mAbs) (Stem Cell Technology) as per the manufacturer's instructions. The CD8-depleted PBMCs were subsequently stimulated with A H3N2 Wyoming, A H3N2 Sydney, and medium control in ELISPOT assays as above. The contribution of CD8 T cells was calculated by subtracting the SFC/106 PBMCs in CD8-depleted PBMCs from the SFC/106 PBMCs in undepleted PBMCs. The results are described as CD8-ELISPOT, whereas the results obtained with undepleted PBMCs are described as total ELISPOT.
Statistical analysis
The medians and 95% confidence limits of the ELISPOT results were calculated using a distribution-free method.25 The comparison between categorical groups was conducted either using the Wilcoxon rank sum test (two groups) or Kruskal–Wallis test (more than two groups). The comparison between different time points was conducted using the Signed rank test.
Results
Baseline characteristics of the study population
Of 243 HIV-infected children enrolled in P1057, ELISPOT results were obtained from 175 (90 in the LAIV group and 85 in the TIV group). There were no differences in demographic or HIV-specific characteristics between the vaccine groups at baseline: the mean age was 11 years, the median CD4 was >25% and >500 cells/μl, and the mean plasma HIV concentration was 2.8 log10 RNA copies/ml in both arms (Table 1) and in all HIV-specific (CD4%) stratification groups (data not shown).
Table 1.
Baseline Characteristics of the Study Population by Treatment Arm
| Parameters | LAIV | TIV |
|---|---|---|
| Subjects | 90 | 85 |
| Age in years [Mean (SD)] | 11.1 (3.3) | 11.6 (2.8) |
| CD4 absolute count [Mean (SD)] | 862 (366) | 940 (379) |
| CD4% [Mean (SD)] | 33.5 (8.6) | 34.4 (8.2) |
| Ethnicity | ||
| White-non-Hispanic | 13 (14%) | 8 (9%) |
| Black-non-Hispanic | 53 (59%) | 58 (68%) |
| Hispanic | 21 (23%) | 17 (20%) |
| Others | 3 (3%) | 2 (2%) |
| Gender | ||
| Male | 52 (58%) | 43 (51%) |
| Female | 38 (42%) | 42 (49%) |
| Log10 plasma HIV RNA [Mean (SD)] | 2.8 (0.7) | 2.8 (0.7) |
| ELISPOT [Median SFC/106 PBMCs (95% CI)] | ||
| A H1N1 New Caledonia | 126 (101; 160) | 190 (156; 246) |
| A H3N2 Wyominga | 111 (56; 132) | 167 (144; 232) |
| CD8-mediated | 95 (72; 126) | 146 (122; 220) |
| B Jilin | 110 (90; 160) | 136 (105; 177) |
| A H3N2 Sydney | 68 (47; 90) | 94 (71; 106) |
| CD8-mediated | 56 (40; 80) | 85 (64; 106) |
| B Yamanashi | 144 (106; 216) | 169 (126; 206) |
| PHA | 89 (62; 124) | 146 (86; 258) |
Indicates significant differences between LAIV and TIV (p = 0.01, Wilcoxon sum rank test).
Despite randomization and the fact that baseline assays were run simultaneously for the two vaccine groups, baseline ELISPOT responses to influenza strains and PHA tended to be higher in TIV than LAIV recipients (Table 1). Differences reached statistical significance only for A H3N2 Wyoming total ELISPOT (medians of 167 vs. 111 SFC/106 PBMCs, respectively; p = 0.01, Wilcoxon rank sum test). CD8 T cells mediated ≥87% of the ELISPOT responses detected in these HIV-infected children on highly active antiretroviral therapy (HAART).
There were no differences in baseline ELISPOT results by HIV-specific (CD4%) stratification groups. The proportions of subjects in groups 1, 2, and 3 that had ELISPOT values >100 SFC/106 PBMCs were 72%, 66%, and 65%, respectively, for A H1N1 New Caledonia; 71%, 62%, and 65%, respectively, for A H3N2 Wyoming; and 51%, 49%, and 60%, respectively, for B Jilin.
ELISPOT responses to TIV
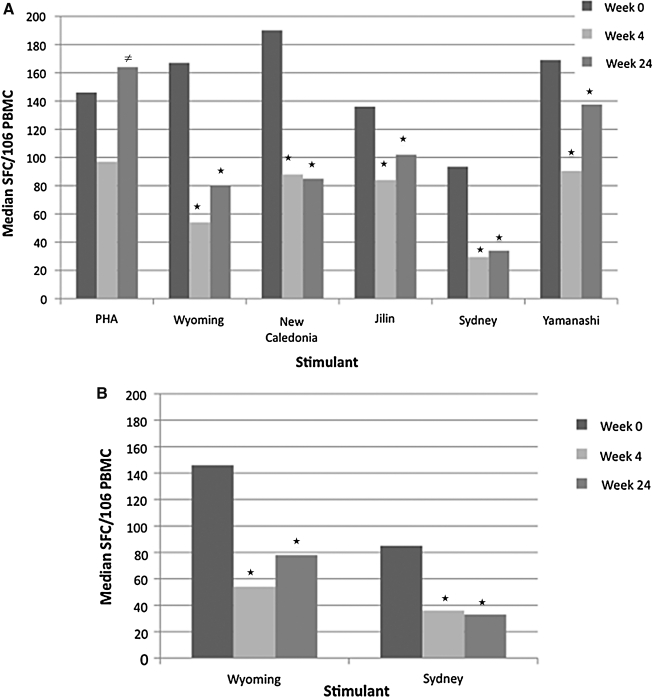
At 4 weeks after TIV, total ELISPOT responses significantly decreased to the three influenza viruses in the vaccine and to the mismatched influenza viruses by 1.5- to 3-fold (p < 0.001; Fig. 1A). There was also a trend to a decrease in ELISPOT responses to PHA (p = 0.06; Fig 1A). The influenza-specific CD8-ELISPOT responses to the two A/H3N2 strains tested also significantly decreased 4 weeks after TIV compared to baseline (p < 0.001; Fig 1B). At 24 weeks, influenza-specific total and CD8-ELISPOT values were significantly lower than baseline (p ≤ 0.03), but PHA responses significantly increased compared with week 4 values (p < 0.01) and returned to levels similar to the baseline levels (Fig. 1).
FIG. 1.
ELISPOT responses of TIV recipients. Data were derived from 85 HIV-infected recipients whose PBMCs were tested by ELISPOT after stimulation with PHA, influenza strains contained in the seasonal vaccine (A H3N2 Wyoming, A H1N1 New Caledonia and B Jilin), and mismatched influenza strains (A H3N2 Sydney and B Yamanashi). Bars represent medians for each group. Asterisks (*) indicate significant differences from baseline and the unequal sign (≠) indicates significant difference from week 4. (A) Total ELISPOT responses representing all PBMCs; (B) CD8 ELISPOT responses representing CD8 cells only. There were significant decreases in total and CD8 ELISPOT responses against all influenza strains at 4 and 24 weeks after vaccination (p ≤ 0.03). There was a trend toward a decrease in PHA-stimulated ELISPOT at 4 weeks after vaccination (p = 0.06) followed by a significant rebound at 24 weeks (p = 0.01).
There were no differences in responses by HIV-specific group (CD4% categorical values), baseline CD4% continuous values, plasma HIV RNA, age, gender, or ethnicity. Similarly, there were no differences in ELISPOT responses by baseline HAI titers, which were previously described.5 CD4% and plasma HIV RNA concentration did not change over time in participants of this study,5 nor were there any changes in CD8 or CD19 cells over time. Therefore, changes in these lymphocyte populations did not explain the decrease of ELISPOT values after vaccination. Since the decrease of ELISPOT results was unexpected, we investigated potential biases that might have been introduced by the geographic location of the subject or by the time when the assay was performed. This was done by showing the absence of clusters of low results by clinical site or date of assay. Moreover, because the study accrued over a period of 2.5 months, there was a considerable time overlap between baseline and week 4 ELISPOT assays in the laboratory, and there was no downward (or upward) trend over time among baseline ELISPOT values, demonstrating the stability of the assay.
ELISPOT responses to LAIV
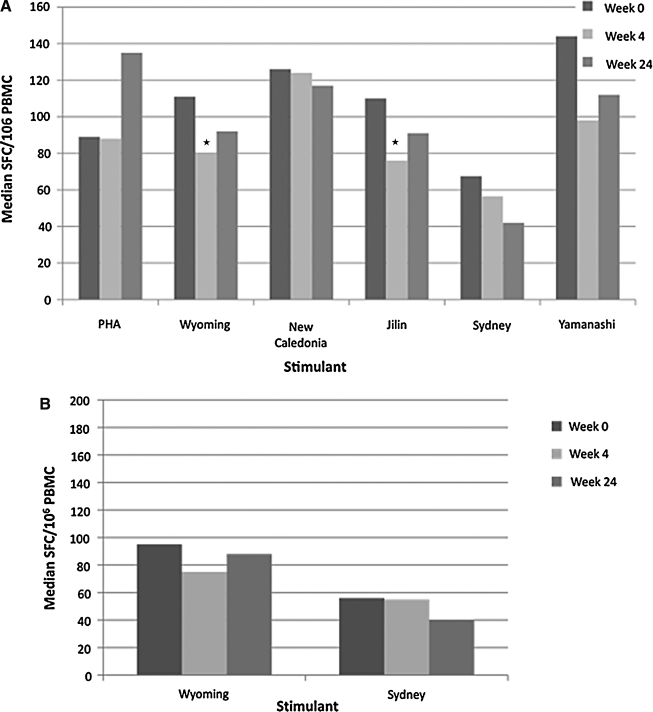
At 4 weeks after LAIV, influenza A H3N2 Wyoming and B Jilin total ELISPOT values decreased by 1.4-fold (p ≤ 0.03; Fig. 2A). Total ELISPOT responses to other influenza strains and to PHA (Fig. 2A), and CD8-ELISPOT responses to A H3N2 influenza viruses (Fig. 2B), did not significantly change compared to baseline. At 24 weeks after LAIV, all ELISPOT responses were similar to those measured at baseline. Analyses of responses to LAIV by the HIV-specific group, baseline CD4%, plasma HIV RNA concentration, age, gender, ethnicity, geographic location of the subject, and date of assay, similar to those described for TIV, did not show any significant associations.
FIG. 2.
ELISPOT responses of LAIV recipients. Data were derived from 90 HIV-infected recipients whose PBMCs were tested by ELISPOT after stimulation with PHA, influenza strains contained in the seasonal vaccine (A H3N2 Wyoming, A H1N1 New Caledonia and B Jilin), and mismatched influenza strains (A H3N2 Sydney and B Yamanashi). Bars represent medians for each group. Asterisks (*) indicate significant differences from baseline. (A) Total ELISPOT responses representing all PBMCs; (B) CD8 ELISPOT responses representing CD8 cells only. There were significant decreases in total ELISPOT responses against influenza strains A H3N2 Wyoming and B Jilin at 4 weeks after vaccination (p ≤ 0.03). All other total and CD8 ELISPOT responses were not significantly different from baseline.
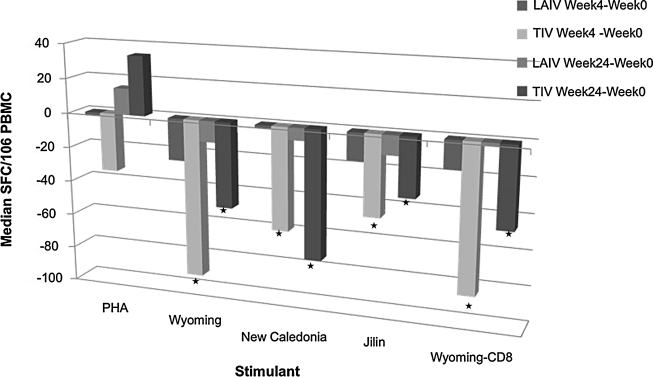
Comparison between ELISPOT responses to LAIV and TIV
Since neither vaccine increased ELISPOT responses to influenza strains, we sought to determine if the decrease in ELISPOT was significantly different after LAIV vs. TIV. Figure 3 shows the comparison between the decrease in ELISPOT responses at 4 and 24 weeks after vaccination compared to baseline. The decreases in total and CD8-ELISPOT responses against vaccine-contained influenza A strains were significantly greater in TIV than in LAIV recipients (p ≤ 0.02). However, since baseline ELISPOT responses tended to be higher at baseline in TIV compared with LAIV recipients, at week 4, the ELISPOT responses were not appreciably different in the two groups. PHA-stimulated nonspecific ELISPOT values tended to have a more pronounced decrease at week 4 after TIV compared with LAIV (p = 0.07). Changes in vaccine-contained influenza B ELISPOT results did not significantly differ between the two treatment groups at 4 and 24 weeks after vaccination.
FIG. 3.
Comparison of ELISPOT decreases of LAIV and TIV recipients from baseline to week 4 after vaccination and from baseline to week 24 after vaccination. Data were derived from 85 and 90 HIV-infected TIV and LAIV recipients, respectively. Bars represent median differences from study week 0 to week 4 or 24. Asterisks (*) indicate significant differences between treatment groups. There were significantly larger differences in total and CD8 ELISPOT responses to all influenza strains in the seasonal vaccine of TIV vs. LAIV recipients (p ≤ 0.02).
Baseline ELISPOT values and shedding of vaccine virus
To gain insight into the association of ELISPOT-measured CMI with protection against influenza, we used the absence of LAIV viral shedding as a surrogate marker for vaccine-induced influenza protection and correlated this end point with baseline ELISPOT values. On day 3 after vaccination, the influenza A H1N1 New Caledonia vaccine strain was recovered from 18 LAIV recipients who participated in the ELISPOT substudy, vaccine strain B was recovered from six subjects, and vaccine strain H3N2 was recovered from two subjects. A comparison of baseline ELISPOT results between vaccine virus shedders and nonshedders was performed for influenza A H1N1 and B, but not for A H3N2, due to the low number of shedders. Baseline influenza A H1N1 median (95% CI) ELISPOT values were 133 (75; 267) and 84 (52; 218) SFC/106 PBMC among nonshedders and shedders, respectively (p = 0.27). For influenza B, corresponding results were 110 (52; 244) and 63 (19; 122) SFC/106 PBMCs (p = 0.3).
Discussion
The HIV-infected children who received TIV experienced a significant decrease in ELISPOT responses to the influenza strains in the vaccine and to mismatched strains. LAIV administration did not decrease the influenza-specific ELISPOT responses of HIV-infected children, but did not increase them either. A generalized decrease of CMI or of influenza-specific CMI has not been reported by other investigators who assessed T cell responses to influenza vaccines administered to healthy individuals.15,18,26,27 The administration of a virosomal influenza vaccine to HIV-infected children on HAART also did not appear to decrease their CMI.28,29 ELISPOT assays are not standardized and there is variability across laboratories,30,31 which may explain the difference between our results and those of others. We systematically sought and eliminated potential technical problems that might have biased our results, such as changes in assay characteristics over time and errors in sample collection and transportation. Moreover, although this study did not include uninfected controls, we previously found that ELISPOT values increased in healthy young adults vaccinated with LAIV or TIV.32 Taken together, these data validate the ELISPOT results.
The mechanism underlying the decrease in influenza-specific ELISPOT results of HIV-infected children after TIV administration is unclear. There are several potential mechanisms unique to HIV infection, including a strong Th2 response to the vaccine that attenuates the Th1 response, and/or stimulation of regulatory cells by the vaccine. The first invoked mechanism seems the most likely, since TIV, which is a stronger antibody inducer than LAIV, also suppresses ELISPOT responses more vigorously. Furthermore, HIV-infected hosts have a bias toward Th2 responses compared with normal hosts,33 which may explain the difference in CMI responses to TIV between HIV-infected and -uninfected individuals. However, we were unable to demonstrate a negative correlation between antibody and CMI responses to TIV. The second hypothesis, invoking T cell regulation, is supported by evidence that HIV-infected individuals have higher frequencies of regulatory T cells.34–37 Antigen presentation by immature dendritic cells may induce regulatory T cells38 and HIV-infected individuals accumulate immature dendritic cells due to their impaired ability to clear these cells.39,40 Recent observations ascribe a regulatory role to activated B cells,41 which is an appealing hypothesis in the scenario of CMI suppression following immunization. It is also possible, although less likely, that ELISPOT responses, which would have been generated in the previous influenza season, were declining at the time of enrollment in this study. If this were the case, administration of TIV did not affect the natural decline of the influenza-specific ELISPOT responses, whereas LAIV stopped it. Further investigation is needed to identify the mechanism responsible for the T cell response to influenza vaccines in HIV-infected children observed in this study.
The clinical significance of the diminished influenza-specific ELISPOT after TIV is unclear. We were unable to demonstrate an association of baseline ELISPOT values with protection against LAIV viral shedding. However, in a large LAIV efficacy trial of healthy children immunized for the first time, Forrest et al.18 observed a significant association between the acquisition of ELISPOT responses ≥100 SFC/106 PBMCs after vaccination and protection against influenza disease. In our study, the baseline ELISPOT values of nonshedders were higher than those of shedders, but the differences demonstrated in our small sample size did not reach statistical significance.
An additional concern is that T cell responses to PHA also tended to decrease at 4 weeks after TIV administration, although they significantly rebounded at 24 weeks. A more global T cell depression could have repercussions on the control of HIV infection or other opportunistic infections. In this study, the plasma HIV RNA levels and the CD4% remained stable overall in the study participants, irrespective of the type of vaccine that they received. However, our study participants were on highly active antiretroviral therapy (HAART) as per inclusion criteria. Before HAART was available, several studies showed increases in plasma HIV RNA after TIV.42–47 This was ascribed to transient CD4 activation, but, perhaps, a transient decrease in CD8 function could also have contributed to this adverse effect of TIV. Retroviral infection of nonhuman primate models demonstrated that CD8 depletion results in a pronounced increase in viral replication.48,49
This study raises an important question regarding CMI responses after TIV administration to HIV-infected individuals. To elucidate the effect of strong antibody inductions on CMI of HIV-infected patients, further studies are needed after the administration of TIV and of other antibody-inducing vaccines. The most important concern is the effect of these vaccines on the CMI of HIV-infected individuals who are not on HAART or whose viral load is not effectively controlled with available antiretroviral therapies.
Acknowledgments
This research was supported by Grant 5 MO1-RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH; N01-HD-3-3162 and by Grants U01AI068632 and U01 AI068616 from the National Institute of Allergy and Infectious Diseases; N01-HD-3-3345 and contract N01-HD-33162 (A.W.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and by MedImmune LLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health. We thank Drs. Robert Walker and Maria Allende for their participation in this study and their critical review of this article.
IMPAACT/PACTG P1057 sites and contributors: Site 3701 Johns Hopkins University Hospital (Beth Griffin, RN; Nancy Hutton, MD; Mary Joyner, NP; Andrea Ruff, MD); 4701Duke University–Pediatric (Joan Wilson, RN; Mary Jo Hassett, RN; Carole Mathison; John Swetnam); 5006 Harlem Hospital (Elaine Abrams, MD; Maxine Frere, RN; LisaGaye Robinson, MD); 5012 NYU Medical Center/Bellevue (William Borkowsky, MD; Sandra Deygoo, BS; Siham Akleh, RN; Aditya Kaul, MD); 5018 University of South Florida Physicians Group (Jorge Lujan-Zilberman, MD; Patricia Emmanuel, MD; Carolyn Graisbery, RN; Carina Rodriguez, MD); 5024 Children's Hospital Kings and Daughters (Randall G. Fisher, MD; Kenji M. Cunnion, MD, MPH; Laura Sass, MD; Donna Sandifer, RN); 5026 Mount Sinai (Mary S. Dolan, RN; Roberto Posada, MD); 5038 Yale University School of Medicine (Warren A. Andiman, MD; Leslie Hurst, BS; Sostena Romano, APRN, MBA); 5040 SUNY Health Science Center (Denise Ferraro, RN; Michele Kelly, PNP; Margaret Oliver, LPN); 5051 University of Florida Health Science Center (Mobeen Rathore, MD; Nizar Maraqa, MD; Kathy Thomas, MA; Angela Lala, LPN); 5052 The Children's Hospital–University of Colorado (Mark Abzug MD; Emily Barr; Megan Canon; Josephine Greenquist); 5057 University of Rochester–Pediatric Component (Geoffrey A. Weinberg, MD; Francis Gigliotti, MD; Barbra Murante, RNC, PNP; Susan Laverty, RN); 5095 Tulane University (Margarita Silio, MD; Thomas Alchediak, MD; Cheryl Borne, RN; Sheila Bradford, RN); 6701 The Children's Hospital of Philadelphia IMPAACT CTU (Steven D. Douglas, MD; Richard M. Rutstein, MD; Carol A. Vincent, CRNP, MSN; Patricia C. Coburn, RN, BSN); 7301 University of Massachusetts Medical School (Katherine Luzuriaga, MD; Richard Moriarty, MD; William (Jerry) Durbin, MD; Donna Picard, RN); 60336 Baylor College of Medicine (Chivon D. McMullen-Jackson, RN, ADN; Theresa Aldape, LMSW; Mary E. Paul, MD; Heidi L. Schwarzwald, MD, MPH); 60422 St. Jude/Memphis (Gregory Storch, MD; Laura Pickering, RN; Katherine Knapp, MD; Jill Utech, RN); 60444 Family Clinical Trials Center (Mavis Dummitt, RN; Caroline Nubel; Stefan Hagmann, MD; Murli Purswani, MD); 2901 Boston Children's Hospital; 3601 UCLA Medical Center; 4001 Children's Hospital of Chicago; 4501 UCSF Medical Center; 4601 UCSD Medical Center; 5008 Children's Hospital at SUNY Downstate; 5013 Jacobi Medical Center; 5031 City Hospital at San Juan; 5041 Children's Hospital of Michigan; 5055 Children's Diagnostic & Treatment Center of South Florida; 5056 University of Florida at Gainesville; 6501 St. Jude Children's Research Hospital; 7701 University of Alabama, Birmingham; 60341 Columbia Collaborative–HIV/AIDS; 60349 University of Miami Pediatric Perinatal HIV/AIDS; 60358 N.J. Medical School.
Disclosure Statement
A.W. has research grants from MedImmune. M.J.L. is a consultant for MedImmune, Novartis, and Glaxo-Smith Kline. M.J.L. has research grants from Glaxo-Smith Kline and Sanofi Pasteur. S.A.N. is a consultant for Wyeth Pharmaceuticals.
Reference
- 1.Malaspina A. Moir S. Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 2.Tasker SA. O'Brien WA. Treanor JJ, et al. Effects of influenza vaccination in HIV-infected adults: A double-blind, placebo-controlled trial. Vaccine. 1998;16:1039–1042. doi: 10.1016/s0264-410x(97)00275-2. [DOI] [PubMed] [Google Scholar]
- 3.King JC., Jr Fast PE. Zangwill KM, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20:1124–1131. doi: 10.1097/00006454-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 4.King JC. Jr, Treanor J.Fast PE, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults J Infect Dis 2000181725–728. [DOI] [PubMed] [Google Scholar]
- 5.Levin MJ. Song LY. Fenton T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26:4210–4217. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhorer J. Ambrose CS. Dickinson S, et al. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009;27:1101–1110. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB. Edwards KM. Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 8.Belshe RB. Gruber WC. Mendelman PM, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181:1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 9.Belshe RB. Mendelman PM. Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM. Dilzer AM. Meents DL. Swain SL. CD4 T cell-mediated protection from lethal influenza: Perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 11.Cerwenka A. Morgan TM. Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: Homing properties rather than initial frequencies are crucial. J Immunol. 1999;163:5535–5543. [PubMed] [Google Scholar]
- 12.Mbawuike IN. Piedra PA. Cate TR. Couch RB. Cytotoxic T lymphocyte responses of infants after natural infection or immunization with live cold-recombinant or inactivated influenza A virus vaccine. J Med Virol. 1996;50:105–111. doi: 10.1002/(SICI)1096-9071(199610)50:2<105::AID-JMV1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Mbawuike IN. Zhang Y. Couch RB. Control of mucosal virus infection by influenza nucleoprotein-specific CD8+ cytotoxic T lymphocytes. Respir Res. 2007;8:44. doi: 10.1186/1465-9921-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElhaney JE. Xie D. Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 15.He XS. Holmes TH. Mahmood K, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–811. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 16.He XS. Holmes TH. Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He XS. Mahmood K. Maecker HT, et al. Analysis of the frequencies and of the memory T cell phenotypes of human CD8+ T cells specific for influenza A viruses. J Infect Dis. 2003;187:1075–1084. doi: 10.1086/368218. [DOI] [PubMed] [Google Scholar]
- 18.Forrest BD. Pride MW. Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042–1053. doi: 10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Orsogna LJ. Krueger RG. McKinnon EJ. French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747–1752. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 20.Moir S. Ho J. Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viau M. Veas F. Zouali M. Direct impact of inactivated HIV-1 virions on B lymphocyte subsets. Mol Immunol. 2007;44:2124–2134. doi: 10.1016/j.molimm.2006.07.302. [DOI] [PubMed] [Google Scholar]
- 22.Miiro G. Kayhty H. Watera C, et al. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis. 2005;192:1801–1805. doi: 10.1086/497144. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg A. Huang S. Fenton T, et al. Virologic and immunologic correlates with the magnitude of antibody responses to the hepatitis A vaccine in HIV-infected children on HAART. J Acquir Immune Defic Syndr. 2009;52:17–24. doi: 10.1097/QAI.0b013e3181b011f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg A. Dickover R. Britto P, et al. Continuous improvement in the immune system of HIV-infected children on prolonged antiretroviral therapy. AIDS. 2008;22:2267–2277. doi: 10.1097/QAD.0b013e3283189bb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn G. Meeker WQ. A Guide for Practitioners. John Wiley & Sons; New York; 1991. Statistical Intervals. [Google Scholar]
- 26.Avetisyan G. Ragnavolgyi E. Toth GT. Hassan M. Ljungman P. Cell-mediated immune responses to influenza vaccination in healthy volunteers and allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:411–415. doi: 10.1038/sj.bmt.1705064. [DOI] [PubMed] [Google Scholar]
- 27.Zeman AM. Holmes TH. Stamatis S, et al. Humoral and cellular immune responses in children given annual immunization with trivalent inactivated influenza vaccine. Pediatr Infect Dis J. 2007;26:107–115. doi: 10.1097/01.inf.0000253251.03785.9b. [DOI] [PubMed] [Google Scholar]
- 28.Tanzi E. Esposito S. Bojanin J, et al. Immunogenicity and effect of a virosomal influenza vaccine on viral replication and T-cell activation in HIV-infected children receiving highly active antiretroviral therapy. J Med Virol. 2006;78:440–445. doi: 10.1002/jmv.20559. [DOI] [PubMed] [Google Scholar]
- 29.Vigano A. Zuccotti GV. Pacei M, et al. Humoral and cellular response to influenza vaccine in HIV-infected children with full viroimmunologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48:289–296. doi: 10.1097/QAI.0b013e3181632cda. [DOI] [PubMed] [Google Scholar]
- 30.Cox JH. Ferrari G. Kalams SA. Lopaczynski W. Oden N. D'Souza M P. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res Hum Retroviruses. 2005;21:68–81. doi: 10.1089/aid.2005.21.68. [DOI] [PubMed] [Google Scholar]
- 31.Janetzki S. Panageas KS. Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammitt LL. Li S. Patterson-Bartlett J, et al. Kinetics of viral shedding, immune responses to cold-adapted influenza vaccine. X International Symposium on Respiratory Viral Infections; Singapore, Thailand: 2008. Feb 28–Mar 2. [Google Scholar]
- 33.Hyjek E. Lischner HW. Hyslop T, et al. Cytokine patterns during progression to AIDS in children with perinatal HIV infection. J Immunol. 1995;155:4060–4071. [PubMed] [Google Scholar]
- 34.Aandahl EM. Michaelsson J. Moretto WJ. Hecht FM. Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson J. Boasso A. Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 36.Eggena MP. Barugahare B. Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 37.Tsunemi S. Iwasaki T. Imado T, et al. Relationship of CD4+ CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 38.Dhodapkar MV. Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 39.Krathwohl MD. Schacker TW. Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 40.Mavilio D. Lombardo G. Kinter A, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tretter T. Venigalla RK. Eckstein V, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008;112:4555–4564. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
- 42.Fuller JD. Craven DE. Steger KA. Cox N. Heeren TC. Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: Impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 43.Glesby MJ. Hoover DR. Farzadegan H. Margolick JB. Saah AJ. The effect of influenza vaccination on human immunodeficiency virus type 1 load: A randomized, double-blind, placebo-controlled study. J Infect Dis. 1996;174:1332–1336. doi: 10.1093/infdis/174.6.1332. [DOI] [PubMed] [Google Scholar]
- 44.Keller M. Deveikis A. Cutillar-Garcia M, et al. Pneumococcal and influenza immunization and human immunodeficiency virus load in children. Pediatr Infect Dis J. 2000;19:613–618. doi: 10.1097/00006454-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien WA. Grovit-Ferbas K. Namazi A, et al. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 46.Stanley SK. Ostrowski MA. Justement JS, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 47.Staprans SI. Hamilton BL. Follansbee SE, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loffredo JT. Burwitz BJ. Rakasz EG, et al. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J Virol. 2007;81:2624–2634. doi: 10.1128/JVI.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz JE. Kuroda MJ. Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]