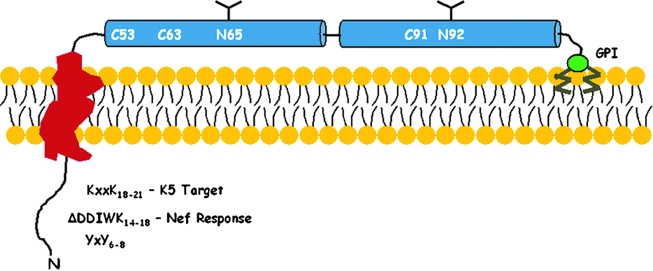
FIG. 1.
Domain structure of BST-2. BST-2 is a type II (N-terminus in the cytoplasm) single-pass transmembrane protein whose C-terminus is attached to the lipid bilayer via a glycosylphosphatidylinositol (GPI) anchor. The cytoplasmic domain contains several conserved features including a YxY motif that binds the plasma membrane clathrin adaptor AP-2, a DDIWK sequence found in nonhuman primates that is required for response to Nef, and lysines (K18 and K21) that are targets for ubiquitination by the K5 protein of KSHV. The extracellular domain contains two α-helical regions (blue) that are predicted to form dimeric coiled-coils. Cysteines that contribute to disulfide-linked homodimerization and N-linked glycosylation sites are indicated.