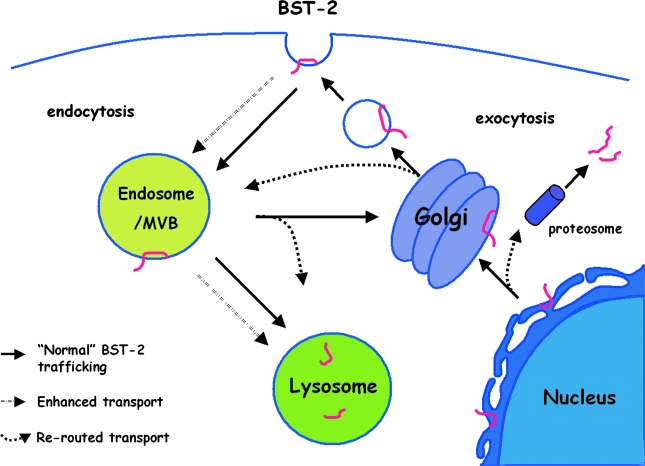
FIG. 4.
Cellular pathways potentially involved in the antagonism of BST-2 via removal from the cell surface by viral proteins. Upon synthesis at the endoplasmic reticulum (ER), BST-2 is normally delivered to the plasma membrane (PM), internalized from the PM via AP-2-dependent endocytosis, and presumably recycled from endosomes to the PM. The viral proteins that decrease the expression of BST-2 at the cell surface (HIV-I Vpu, HIV-2 and SIV Env, KSHV K5, and SIV Nef ) and that decrease the total cellular expression of BST-2 (Vpu and K5) may act at various steps. Certain mechanisms would block newly synthesized BST-2 from reaching the PM. For example, during exocytosis, BST-2 could be rerouted from the ER to the proteosome for degradation (possibly for Vpu) or rerouted from the Golgi to endosomes and lysosomes. BST-2 could also be sequestered after synthesis in the trans-Golgi network (possibly for Vpu and HIV-2 and SIV Env). Alternative mechanisms would directly remove BST-2 from the plasma membrane. For example, the rate of endocytosis of BST-2 could be enhanced (likely for SIV Nef and possibly for HIV-2 and SIV Env). Alternatively or in addition, BST-2 could be rerouted after endocytosis to late endosomes/multivesicular bodies (MVB) with further trafficking to the lysosome (likely for Vpu and K5). The latter pathway would be at the expense of recycling to the cell surface. Mechanisms involving the direct removal of BST-2 from the PM are appealing mechanistically. They are potentially the most rapid, because, unlike mechanisms in which newly synthesized BST-2 is prevented from reaching the PM, they do not rely on the endogenous rate of turnover at the cell surface to clear BST-2 from its site of action as a tethering factor. See text for references.