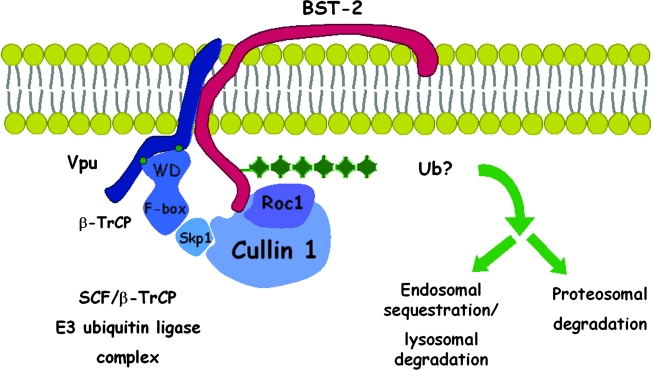
FIG. 5.
Vpu recruits a multisubunit ubiquitin ligase complex to BST-2. Vpu interacts with BST-2 via its transmembrane domain, while recruiting an SCF-E3 multisubunit ubiquitin ligase complex via a conserved DSGxxS motif in its cytoplasmic domain (green dots). The DSGxxS sequence binds β-TrCP, the substrate adaptor for the complex, via its WD domain. The presumed consequence of this interaction is the ubiquitination of BST-2, which may lead to endocytosis, endosomal sequestration, lysosomal degradation, and/or proteasomal degradation. Any of these mechanisms would remove BST-2 from the cell surface, it site of activity as a tethering factor, and so counteract restriction virion release. See text for references.