Figure 2. Examination of the interactions between epitope-tagged LRBP and prey proteins in vitro.
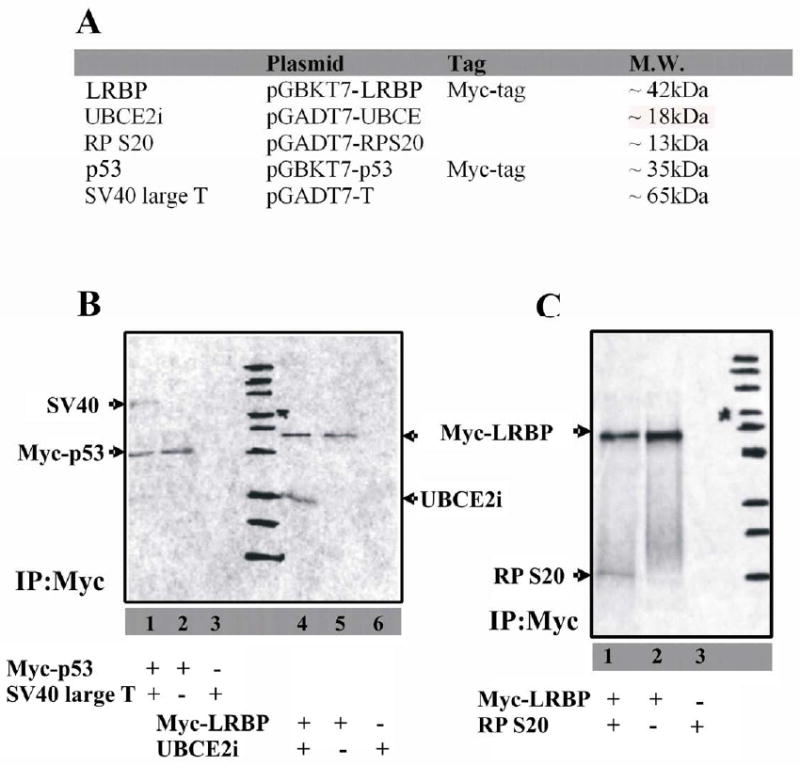
(A) List of proteins synthesized with [35S]-Methionine in vitro. Proteins expressed in pGBKT7 plasmids (LRBP and p53) are Myc tagged.
(B) Proteins were in vitro synthesized in the presence of 35S-methionine. Freshly synthesized LRBP and UBCE2i were mixed together and coimmunoprecipitated with anti-Myc antibody (lane4). The precipitated complex was analyzed by SDS-PAGE and autoradiography. Immunoprecipitation was specific (lane5, LRBP only) and no cross reaction was observed (lane6, UBCE2i only). The p53-SV40 interaction was used as a positive control (lane 1-3). Position of each protein ladder band was marked on the film.
(C) Proteins were in vitro synthesized in the presence of 35S-methionine. LRBP and RP S20 were coimmunoprecipitated with anti-Myc antibody (lane1) and analyzed by SDS-PAGE and autoradiography. Immunoprecipitation was specific (lane2, LRBP only) and no cross reaction was observed (lane3, RPS20 only). Position of each protein ladder band was marked on the film.
