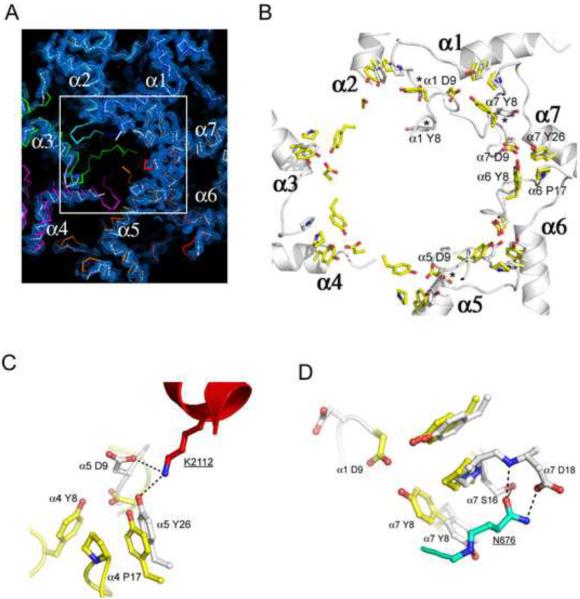
Figure 2. Proteasome conformational changes induced by Blm10.
(A) Top view of the proteasome pore region with electron density for the Blm10 complex. The absence of density for the N-terminal residues of proteasome α2, α3, and α4 indicates that they are disordered in the Blm10 complex (white), whereas they are ordered in the closed, unliganded conformation (colors) and in the fully open complex with PA26 (not shown in this panel).
(B) Open conformation seen in complexes with PA26 (yellow) and Blm10 (white). The stabilizing cluster residues (Tyr8, Asp9, Pro17, Tyr26; (Forster et al., 2003)) are labeled for the α6/α7 cluster, which is ordered in the unliganded proteasome (Groll et al., 1997) and in both the PA26 and Blm10 complexes shown here. Tyr8 and Asp9 residues are not ordered for α2, α3, or α4 in the Blm10 complex. Residues indicated with an asterisk are ordered in the Blm10 complex but are displaced from the open conformation seen with PA26. A version of this panel that also includes the closed conformation is shown in Figure S2.
(C) Contacts that stabilize α5Asp9 away from the open conformation.
(D) Contacts that stabilize α7Tyr8 away from the open conformation.