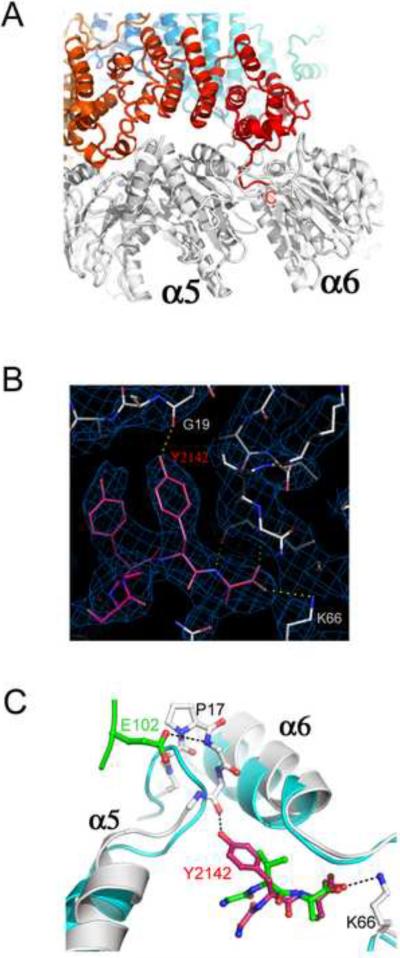
Figure 3. Interactions of the Blm10 C-terminal residues.
(A) Side view with Blm10 C-terminus labeled “C”.
(B) The electron density map is well defined for the Blm10 penultimate tyrosine (Tyr2142) and surrounding residues.
(C) The last three residues of PA26 (green) and Blm10 (red) are shown after overlap of the two complexes on surrounding proteasome residues. Unliganded proteasome (Groll et al., 1997), cyan. Blm10 Tyr2142 stabilizes the open position of α5 by hydrogen bonding with Gly19 O. PA26 stabilizes the same transition by hydrogen bonding interactions of its activation loop residue Glu102.