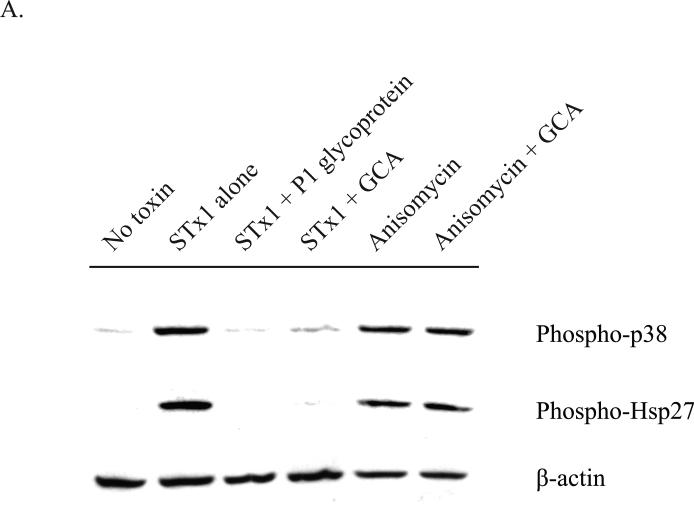
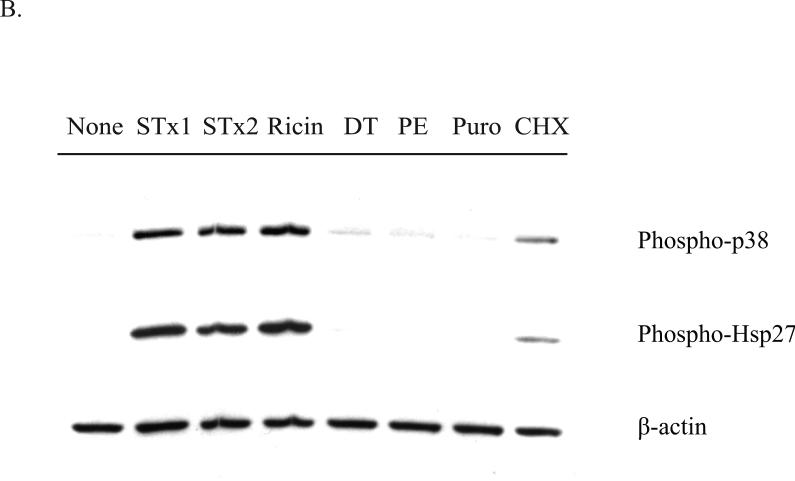
Fig. 5.
Activation of the p38-MK2 pathway by STx depends on toxin adherence and intracellular trafficking and appears to be part of a ribotoxic stress response.
A. HMVEC were pretreated with DMSO (0.5% v/v), GCA (10 μM), or P1 glycoprotein (1 μg/mL) for 30 min prior to exposure to STx1 (10 ng/mL) or media alone (“No Toxin”). Cells were allowed to internalize toxin for 2 h at 37°C prior to lysis and probing with the indicated antibodies. Pretreatment with the P1 glycoprotein inhibited STx1-mediated phosphorylation of p38 and Hsp27, and GCA treatment similarly blocked activation of the p38-MK2 pathway compared to DMSO-treated cells (“Toxin alone”). Pretreatment with GCA, however, had no effect on activation of this pathway following a 2-h exposure to anisomycin (10 ng/mL).
B. HMVEC were exposed to various translational inhibitors for 2 h, and the phosphorylation status of p38 and Hsp27 was assessed by Western blotting. Only inhibitors that are known to cause direct damage to the ribosome (STx1, STx2, and ricin) induced p38 and Hsp27 phosphorylation, while translational inhibitors acting through different mechanisms (DT, PE, Puro, CHX) showed little to no activation. “None” refers to cells lacking compound and toxin treatment. STx1, Shiga toxin 1 (10 ng/mL); STx2, Shiga toxin 2 (10 ng/mL); GCA, Golgicide A; DT, diphtheria toxin (1 μg/mL); PE, Pseudomonas exotoxin A (1 μg/mL); Puro, puromycin (10 μg/mL); CHX, cycloheximide (100 μg/mL). For both (A) and (B), actin staining served as a loading control.