Abstract
Purpose
Previous studies have suggested that higher levels of physical activity may lower lung cancer risk; however, few prospective studies have evaluated lung cancer mortality in relation to cardiorespiratory fitness (CRF), an objective marker of recent physical activity habits.
Methods
Thirty-eight thousand men, aged 20 to 84 years without history of cancer, received a preventive medical examination at the Cooper Clinic in Dallas, TX, between 1974 and 2002. CRF was quantified as maximal treadmill exercise test duration and was grouped for analysis as low (lowest 20% of exercise duration), moderate (middle 40%), and high (upper 40%).
Results
A total of 232 lung cancer deaths occurred during follow-up (mean=17 years). After adjustment for age, examination year, BMI, smoking, drinking, physical activity, and family history of cancer, hazard ratios (95% confidence intervals) for lung cancer deaths across low, moderate and high CRF categories were: 1.0, 0.48 (0.35–0.67), and 0.43 (0.28–0.65) respectively. There was an inverse association between CRF and lung cancer mortality in former (P for trend = 0.005) and current smokers (P for trend <0.001), but not in never smokers (trend P = 0.14). Joint analysis of smoking and fitness status revealed a significant 12-fold higher risk of death in current smokers (HR: 11.9; 95% CI: 6.0–23.6) with low CRF as compared with never smokers who had high CRF.
Conclusions
Although the potential for some residual confounding by smoking could not be eliminated, these data suggest that CRF is inversely associated with lung cancer mortality in men. Continued study of CRF in relation to lung cancer, particularly among smokers, may further our understanding of disease etiology and reveal additional strategies for reducing its burden.
Keywords: Death from lung cancer, physical activity, smoking, prevention, epidemiology
INTRODUCTION
Physical inactivity is associated with an increased overall risk of cancer mortality (17) and mortality associated with specific anatomic sites such as colon (31) and breast (14). However, there is little information regarding the association of inactivity and lung cancer, which is the most common cause of cancer death in the United States. According to the most recent report from the American Cancer Society, in 2009, an estimated 116,090 new cases of lung cancer will be diagnosed and approximately 88,900 men are expected to die from this disease (1). Cigarette smoking is the most important cause of lung cancer. Still, many non-smokers die of the disease, and former smokers remain at elevated risk after quitting. It is estimated in the United States alone, about 3000 lung cancer deaths occur each year in non-smoking adults (1). It takes up to 20 years for majority of former smokers rates to drop to those of never smokers (19). Therefore, it is plausible that other factors besides smoking may play an important etiologic role. Moreover, the majority of cigarette smokers do not develop lung cancer and this fact adds to the likelihood that there may be other factors besides smoking that modify risk. One of these other factors might be physical activity.
Most previous cohort studies (12, 26, 39, 20, 9, 3, 5, 34, 21, 32) have reported an inverse association between risk of lung cancer and physical activity in men, however, some have not (11, 43, 25, 7, 30, 33). These inconsistent findings may be due partly to the measurement errors inherent in self-reported physical activity. Cardiorespiratory fitness (CRF), an objective and more reproducible measure that reflects the functional consequences of physical activity habits, may provide a better exposure with which to evaluate associations with lung caner risk. To the best of our knowledge, only one study (38) has been conducted on CRF and lung cancer mortality among men. However, this study examined only men with pre-diabetes and diabetes. To address cancer prevention strategies, it is important to investigate whether physical activity or CRF reduces cancer incidence or mortality in the general population prospectively. The objective of this report is to examine the risk of lung cancer mortality across levels of fitness, obtained by maximal exercise test on a treadmill, in a large cohort of men from the Aerobics Center Longitudinal Study (ACLS) (17).
METHODS
Study population
The ACLS is a prospective study composed of patients who received preventive medical examinations at the Cooper Clinic in Dallas, Texas. The current analysis included 38,000 men ranging in age from 20 to 84 years who completed a clinical examination including fitness testing between 1974 and 2002 with mortality follow-up through December 31, 2003. Men with any physician-diagnosed cancer or those unable to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate (220 minus age in years) were excluded. Women also were excluded from this analysis due to limitations in sample size and, concomitantly, lung cancer deaths. Most participants were white (>95%) and employed or previously employed in professional occupations. This study was reviewed and approved annually by the Cooper Institute Institutional Review Board, details of which have been published previously (17).
Baseline examination
Participants provided written informed consent to participate in the examination and follow-up study. All medical evaluations included personal and family histories, a questionnaire on demographic characteristics and health habits, a physical examination, anthropometry, electrocardiogram, blood chemistry analyses, blood pressure measurements, and a maximal exercise test on a treadmill. The comprehensive medical evaluation is described in detail elsewhere (38, 17). Briefly, body mass index (BMI) was calculated as measured weight in kilograms (kg) divided by height in meters squared. Based on self-reported current and past smoking behavior, participants were categorized into one of three groups: those who currently smoked cigarettes (current smokers); those who previously smoked cigarettes (former smokers) and those who never smoked cigarettes (never smokers). Number of cigarette smoked, year started smoking, and year quitted smoking were used to calculate pack-years. To measure alcohol use, one unit of alcohol was defined as 12 ounces (3.41 dL) of beer, 5 ounces (1.421 dL) of wine, or 1.5 ounces (0.4262 dL) of heard liquor. Physically inactive was defined as reporting no leisure-time physical activity such as walking, jogging, running, treadmill exercise, cycling, stationary cycling, swimming, racquet sports, aerobic dance, or other sports related activities (e.g., basketball or soccer) in the 3 months before the baseline examination. Family history (from parents and siblings; first-degree relatives) of cancer was obtained from a standardized questionnaire.
Pulmonary function assessment was performed in a subset of the participants (79% of the total study sample) and forced expiratory volume in 1 second (FEV1) was obtained with a Collins 421 Survey spirometer as described elsewhere (8). All procedures were administered by trained technicians who followed standardized protocols. Hankinson et al.(13) derived predictive equations for FEV1 specific for sex, age and height and derived from healthy NHANES-III participants that were used. The FEV1 was expressed both as raw values and as percentage of the predictive values.
We determined CRF using a modified Balke maximal exercise test, as described in previous publications (6, 17). The treadmill speed was 88m • min−1 for the first 25 min. During this time the grade was 0% for the first minute, 2% the second minute and increased 1% each minute until 25 min had elapsed. After 25 min, the grade remained constant while the speed increased 5.4 m • min−1 each minute until test termination. Patients were encouraged to give a maximal effort during the test. The mean (SD) percentage of age-predicted maximal heart rate achieved during exercise was 101.5 (6.6). Total time of the test correlates highly (r = 0.92) with measured maximal oxygen uptake (23). Thus, CRF in this study is analogous to maximal aerobic power. Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake • kg−1 • min−1) were estimated from the final treadmill speed and grade (4). We assigned men to age-specific fitness categories based on their total time on the treadmill test. We classified the lowest 20% as low fit, the next 40% of the fitness distribution as moderately fit, and the upper 40% as high fit, as in our previous reports, based on data from the entire cohort. The detailed cutpoints of treadmill duration and corresponding MET values have been reported earlier (36).
Ascertainment of lung cancer death
All participants were followed from the date of their baseline examination until their date of death or December 31, 2003. The National Death Index (NDI) was the primary data source for mortality surveillance. The underlying cause of death was determined from the NDI report or by a nosologist’s review of official death certificates obtained from the department of vital records in the decedent’s state of residence. Lung cancer mortality was defined by the International Classification of Diseases, Ninth Revision (ICD-9) codes 162.2 to 162.9 before 1999 and Tenth Revision (ICD-10) codes C34 during 1999–2003. We computed person-years of exposure as the sum of follow-up time among decedents and survivors.
Statistical analysis
Baseline characteristics of the study participants were calculated for the entire cohort and by CRF groups. Differences in covariates among the three fitness groups were assessed using F-tests with two degrees of freedom. Kaplan-Meier plots were used to compare survival curves and Cox proportional hazards models were used to compute adjusted hazard ratios (HRs), associated 95% confidence intervals (CIs), mortality rates (deaths/10,000 person-years of follow-up), and linear trends of lung cancer mortality for levels of each fitness category. When calculating HRs, the low-fitness group was used as the reference category. Multivariable-adjusted models controlled for the potential confounding effects of baseline age (years), year of examination, BMI (kg/m2), smoking status (never, former, or current smoker), alcohol intake (drinks per week), physically inactive (yes or no), and family history of cancer (present or not). Tests of linear trend across increasing categories of fitness were conducted by treating the CRF exposure as a single continuous variable. Cumulative hazard plots grouped by exposure suggested no appreciable violations of the proportional hazards assumption.
We also conducted Cox regression analyses of CRF stratified by categories of smoking status (never, former, or current smoker) and by lung function (FEV1/FVC >70% or ≤70%) to assess whether the associations were stronger in particular subgroups. Finally, we examined the joint associations of CRF and smoking status with lung cancer mortality. We assessed the interaction among exposure groups using likelihood ratio tests of nested models. Since smoking is such a strong predictor of lung cancer risk, we further controlled the pack-years smoking in a subset of men who had the information available to calculate this variable. All P-values were two-tailed, and values of less than 0.05 were considered to indicate statistical significance. Analyses were done using SAS statistical software, version 9.1 (SAS Inc., Cary, NC).
RESULTS
The baseline characteristics of participants across levels of fitness are provided in Table 1. Men in the high-fitness group were more likely to have a lower BMI, to have more favorable lipid and blood pressure profiles, to be nonsmokers, and to have higher respiratory function (all P < 0.001).
Table 1.
Baseline characteristics of the study participants across cardiorespiratory fitness (CRF) levels in men, Aerobics Center Longitudinal Study, Dallas, Texas, 1974–2002
| Cardiorespiratory Fitness | |||||
|---|---|---|---|---|---|
| Characteristic | All (n=38000) |
Low CRF (n=6245) |
Moderate CRF (n=15024) |
High CRF (n=16731) |
P for trend |
| Age, years | 43.6 ±9.6 | 42.9 ±9.1 | 43.9 ±9.5 | 43.6 ±9.9 | <0.001 |
| Body mass index, kg/m2 | 26.3 ±3.7 | 29.2 ±5.0 | 26.7 ±3.3 | 24.9 ±2.5 | <0.001 |
| Maximal METs | 11.7 ±2.5 | 8.6 ±1.2 | 10.7 ±1.1 | 13.7 ±1.9 | <0.001 |
| Treadmill time duration, minutes | 18.0 ±5.1 | 11.3 ±2.5 | 16.0 ±2.5 | 22.3 ±3.5 | <0.001 |
| Lipids, mmol/L | |||||
| Total cholesterol | 5.45 ±1.12 | 5.70 ±1.08 | 5.54 ±1.03 | 5.27 ±1.18 | <0.001 |
| HDL-C | 1.17 ±0.32 | 1.03 ±0.27 | 1.11 ±0.28 | 1.25 ±0.33 | <0.001 |
| Triglycerides | 1.56 ±1.29 | 2.13 ±1.74 | 1.67 ±1.21 | 1.23 ±1.04 | <0.001 |
| Fasting blood glucose, mmol/L | 5.60 ±2.84 | 5.84 ±1.46 | 5.59 ±0.93 | 5.50 ±1.12 | <0.001 |
| Blood pressure, mmHg | |||||
| Systolic | 122 ±14 | 124 ±14 | 121 ±13 | 120 ±13 | <0.001 |
| Diastolic | 81 ±10 | 84 ±10 | 82 ±10 | 79 ±9 | <0.001 |
| Cigarette smoking, % | <0.001 | ||||
| Never | 47.6 | 34.6 | 44.7 | 55.1 | |
| Former | 34.4 | 32.8 | 34.5 | 34.9 | |
| Current | 18.0 | 32.6 | 20.8 | 10.0 | |
| Alcohol intake, drinks/week | 4.7 ±6.9 | 3.9 ±7.0 | 4.5 ±7.0 | 5.2 ±6.8 | <0.001 |
| Physically inactive, % | 31.8 | 65.5 | 39.4 | 12.4 | <0.001 |
| FEV1,* liter | 3.8 ±0.7 | 3.6 ±0.7 | 3.8 ±0.7 | 4.0 ±0.6 | <0.001 |
| FEV1 predicted,* liter | 4.2 ±0.5 | 4.1 ±0.4 | 4.2 ±0.5 | 4.2 ±0.5 | <0.001 |
| FEV1% predicted* | 92.4 ±13.7 | 85.9 ±14.3 | 91.4 ±13.3 | 96.0 ±12.6 | <0.001 |
| FVC,* liter | 4.9 ±0.8 | 4.5 ±0.8 | 4.9 ±0.8 | 5.1 ±0.8 | <0.001 |
| FEV1/FVC%* | 78.2 ±7.2 | 78.3 ±7.9 | 78.2 ±7.2 | 78.2 ±7.0 | 0.49 |
| Family history of cancer, % | 1.0 | 0.7 | 1.1 | 1.1 | 0.04 |
Data shown as Means ± SD unless specified otherwise.
METs= maximal metabolic equivalents achieved during the treadmill test; HDL-C= high density lipoprotein cholesterol; FEV1= forced expiratory volume in the first second; FVC= forced vital capacity.
Data only available in 30,185 men.
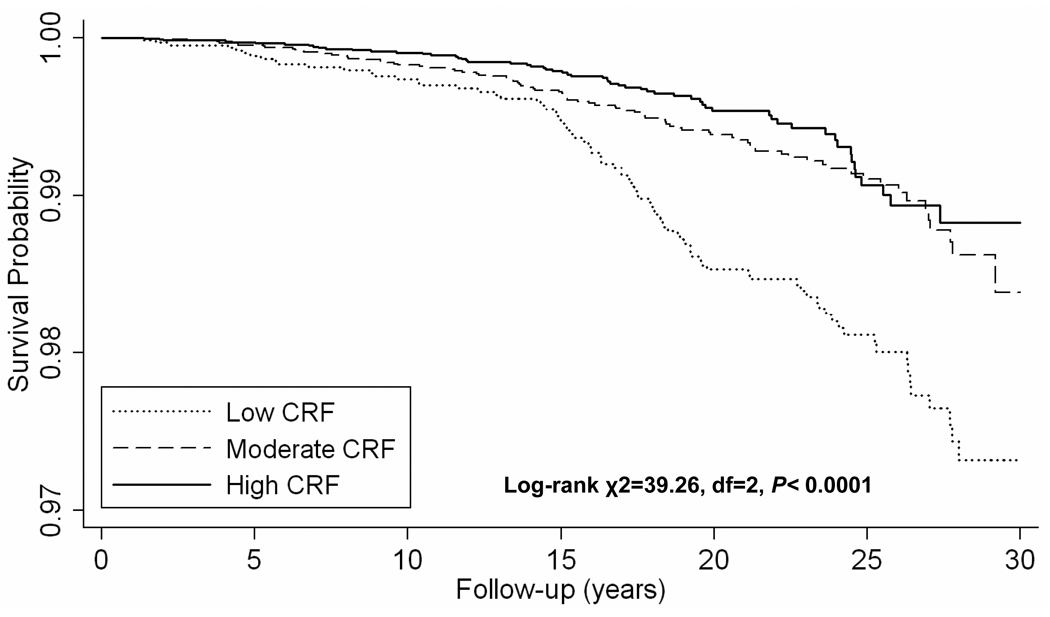
There were 232 deaths from lung cancer during an average 17.1 years of follow-up (649,800 person-years of observation). The risk of lung cancer mortality is lower across incremental levels of fitness (Table 2). After adjustment for covariates (age, examination year, smoking status, alcohol intake, physically inactive, BMI, and family history of cancer), men with moderate and high CRF had 52% and 57% lower lung cancer risk, respectively, than did men with low CRF (P for trend <0.001). The Kaplan-Meier survival curves also indicate that men with moderate and high CRF had greater lung cancer-free time as compared with men with low CRF (Figure 1).
Table 2.
Event rates and hazard ratios for lung cancer mortality by cardiorespiratory fitness (CRF) groups, Aerobics Center Longitudinal Study, Dallas, Texas, 1974–2003
| Deaths for lung caner | Event rate* | HR† | 95% CI† | HR‡ | 95% CI‡ | |
|---|---|---|---|---|---|---|
| All men (n=38,000) | ||||||
| Low CRF | 86 | 7.3 | 1.00 | Referent | ||
| Moderate CRF | 86 | 3.1 | 0.48 | 0.35, 0.67 | ||
| High CRF | 60 | 2.3 | 0.43 | 0.28, 0.65 | ||
| P linear trend | <0.001 | <0.001 | ||||
| Never smoker (n=6,245) | ||||||
| Low CRF | 7 | 2.0 | 1.00 | Referent | ||
| Moderate CRF | 15 | 1.3 | 0.93 | 0.29, 2.96 | ||
| High CRF | 13 | 1.0 | 0.76 | 0.21, 2.79 | ||
| P linear trend | 0.14 | 0.62 | ||||
| Former smoker (n=15,024) | ||||||
| Low CRF | 29 | 7.3 | 1.00 | Referent | ||
| Moderate CRF | 35 | 3.4 | 0.44 | 0.26, 0.74 | ||
| High CRF | 33 | 3.3 | 0.44 | 0.24, 0.81 | ||
| P linear trend | 0.005 | 0.02 | ||||
| Current smoker (n=16,731) | ||||||
| Low CRF | 50 | 12.1 | 1.00 | Referent | ||
| Moderate CRF | 36 | 6.3 | 0.48 | 0.30, 0.76 | ||
| High CRF | 14 | 5.1 | 0.38 | 0.18, 0.79 | ||
| P linear trend | <0.001 | 0.001 | ||||
HR= hazard ratio; CI= conference interval; CRF= cardiorespiratory fitness; BMI= body mass index.
Event rate is expressed as per 10,000 person-years and adjusted for age.
adjusted for age, examination year, smoking status (never, past, or current), alcohol intake (drinks per wk), physical inactivity (yes or no), body mass index (kg/m2), and family history of cancer (present or not).
adjusted for age, examination year, cigarettes per day (for former and current smoker), alcohol intake (drinks per wk), physical inactivity (yes or not), body mass index (kg/m2), and family history of cancer (present or not).
Figure 1.
Kaplan-Meier survival curves for lung cancer mortality by cardiorespiratory fitness (CRF) levels, Aerobics Center Longitudinal Study, Dallas, Texas, 1974–2003.
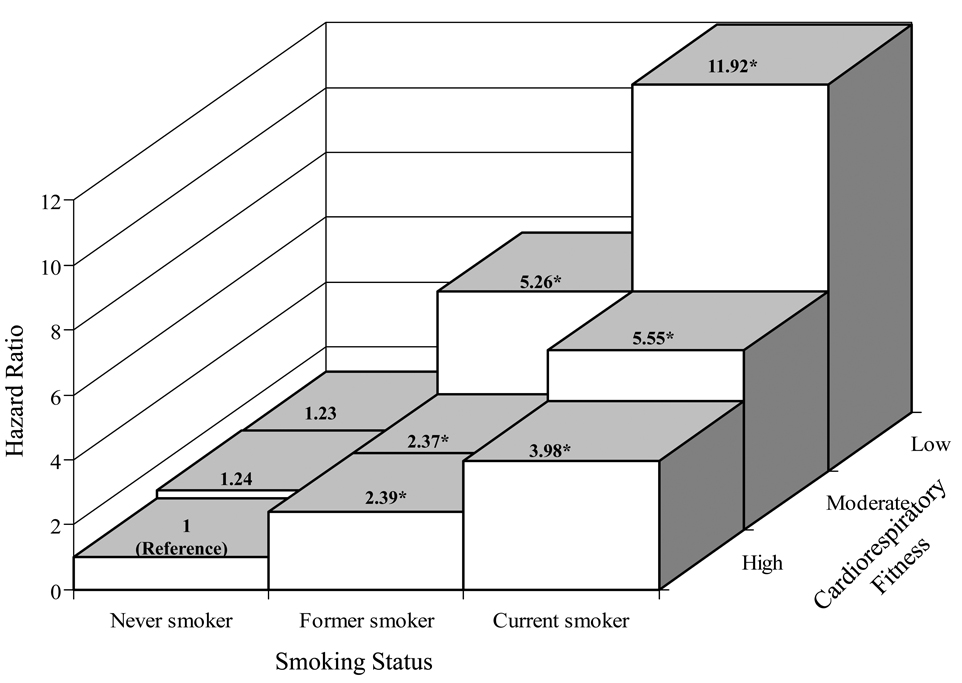
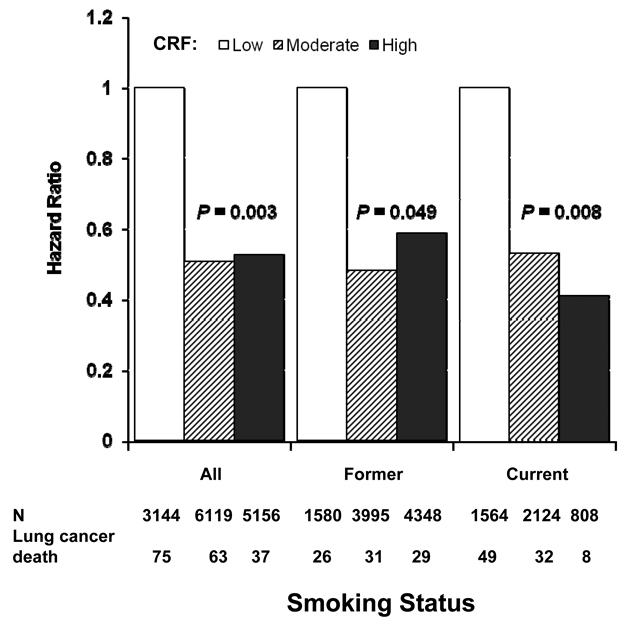
Although there was no significant interaction of CRF with smoking status (P = 0.86), we were interested in examining the smoking-specific association between CRF and lung cancer mortality (Table 2, Figure 2 and 3). The age-adjusted death rate was inversely related to CRF in former smokers (P for trend =0.005) and current smokers (P for trend <0.001), but not in never smoker (P for trend =0.14). Associations attenuated but remained significant within former and current smokers after adjustment for covariates plus cigarettes smoked per day. Excluding deaths during the first 5-years of follow-up did not materially change the magnitude and pattern of the association. Figure 2 show the multivariate-adjusted HRs for lung cancer mortality among nine smoking-fitness combination categories. The highest relative risk was in the category of current smokers with low CRF. This group of men had an almost 12-fold higher risk of dying from lung cancer compared with those never smokers having high fitness (HR 11.92 [95% CI 6.03–23.58]). We further assessed the effect of pack-years smoking on associations between fitness and lung cancer risk in a subset of smokers (N=14,419) who had the data available for us to calculate pack-years smoking (Figure 3). Additional adjustment for pack-years smoking in this subset slightly attenuated the association between CRF and lung cancer mortality, but the pattern of the associations did not materially change. Among men smoking 20 pack-years or more, a lower lung cancer mortality risk was observed among those men who were at least moderate fit (HR 0.49 [95% CI 0.38–0.85]) compared with low fit men.
Figure 2.
Multivariate risk for lung cancer mortality by smoking status and fitness level. The height of bars represent hazard ratios adjusted for age, examination year, alcohol use, BMI, physical activity, and family history of cancer.
Figure 3.
Multivariate-adjusted hazard ratios for cardiorespiratory fitness (CRF) and lung cancer mortality in a subset of men (N=14,419) who had available data to calculate the pack-years in the Aerobics Center Longitudinal Study. The height of bars represent hazard ratios adjusted for age, examination year, pack-years smoking, alcohol use, BMI, physical activity, and family history of cancer.
Finally, we examined the influence of lung function on the association between fitness and lung cancer risk in a large subgroup of men (N=30,185). There was an inverse gradient for the risk of lung cancer mortality across levels of fitness in lower (P for trend =0.008) and higher (P for trend =0.03) lung function groups. Among men with lower lung function, risk was lower in the moderate- (HR 0.67[95% CI 0.37–1.20]) and high- (HR 0.38 [95% CI 0.16–0.89]) CRF groups. In individuals with higher lung function, risk was lower in both the moderate- (HR 0.44[95% CI 0.28–0.68]) and high- (HR 0.50 [95% CI 0.29–0.86]) CRF groups.
DISCUSSION
The primary finding of this study was that higher levels of fitness were associated with a lower risk of lung cancer mortality in men. Compared with smokers, nonsmokers had the lowest risks of lung cancer mortality regardless of their fitness levels. Our data also support the hypothesis that CRF may be protective for lung cancer mortality in current and former smoking men. These associations persisted after controlling for potential confounders. To the best of our knowledge, only one previous study has assessed the association of CRF with risk of dying of lung cancer (38). In that study, Thompson et al.(38) found diabetic and pre-diabetic men who were fit, as defined by achieving at least a moderate level of fitness during a maximal exercise test, had a 57% lower risk of lung cancer mortality. In our study, we found that men with at least a moderate fitness level had a 52% lower lung cancer risk than did men with low CRF.
Our findings are consistent with evidence from previous cohort studies in men examining lung cancer incidence (12, 26, 39, 20, 9, 3, 5, 34, 21, 32). Most of these studies combined fatal and nonfatal lung cancer endpoint as outcome (26, 39, 20, 9, 5, 34, 21, 32), very few used only mortality data (12, 3). A recent meta-analysis also concluded that higher levels of leisure-time physical activity protect against lung cancer (37). In a large study published in 1997 by Thune and Lund, a significant inverse relationship between activity and risk of lung cancer was found (39). After appropriate adjustment for potential confounders, only leisure activity was associated with a lower risk, and only in men. Analysis of data in smokers considered separately showed a significant association between inactivity and lung cancer risk. However, the small number of lung cancer cases in non-smokers and former smokers precluded separate analysis in those groups (39). The Harvard Alumni Health Study also reported a decrease in lung cancer risk in men (20). An energy expenditure of 12 600 kJ/week had a 39% lower risk of lung cancer compared with the reference group (< 4200 kJ/week). These findings were significant when non-smokers and former smokers were considered separately. The trend for smokers was similar, but the results were not statistically significant, perhaps due to small numbers. In addition, Garfinkel and Spellman reported a lower incidence of lung cancer death at higher levels of leisure and occupational activity in 868,000 in both smokers and non-smokers participating in the American Cancer Society’s Cancer Prevention Study II (12). In contrast to the earlier studies, Leitzmann et al.(21) reported no association between physical activity and total lung carcinoma among never smokers, but an inverse association among both former and current smokers. In agreement with this study, we found a similar pattern of the association among never, former and current smokers. Besides the above studies, most of the other studies that found an inverse association between activity and lung cancer risk did not conduct subgroup analyses in current and former smokers (26, 9, 3, 5, 34, 21, 32).
The lack of association in never smokers in our study may be explained partly by the small number of lung cancer deaths. Since we noted only a slight attenuation of the relation between CRF and lung cancer mortality after controlling for pack-years of smoking, residual confounding by cigarette smoking seems unlikely. Another possible explanation might be the potential different etiology of lung cancer between never smokers and smokers (42, 35). It is known, for example, that smoking is more strongly related to squamous cell than adenocarcinomas (16). Several etiologic factors have been proposed for the development of lung cancer in the never smokers, including exposure to radon, cooking fumes, asbestos, heavy metals, and environmental tobacco smoker, human papillomavirus infection, and inherited genetic susceptibility (35). The different biology of lung cancer in never smokers is apparent in differential responses to epidermal growth factor receptor inhibitors and an increased prevalence of adenocarcinoma history in never smokers (42). However, there is still lack of a clear understanding of the factors responsible for lung cancer in never smokers. Future studies should have sufficient numbers of histopahtological subtypes to allow separate analyses.
Clearly, the most important predictor of lung cancer is smoking, though it is more important in squamous cell cancers. Could the increased risk in the low-fit group be the result of unreported smoking in that group, rather than low fitness? This is unlikely for several reasons. Firstly, the association of lower mortality across fitness groups holds for current smokers among whom, by definition, smoking would not be underreported. Secondly, adjustments were made carefully for current and former smoking behavior, number of cigarettes smoked daily, as well as for pack-years smoking. After adjustment for cigarette smoking in the main analyses and adjustment for pack-years smoking in a subset of men, we found similar results. In addition, among men smoking 20 pack-years or more, a reduced lung cancer mortality risk was observed among those who were at least moderate fit compared to low-fit men. Therefore, it is unlikely that the results of the present study reflect confounding by cigarette smoking. The data from the Norway study provide another reason that it is unlikely that the observed inverse association between activity or fitness and decreased risk of lung cancer is due to unreported smoking in a low activity or fit group (39). In this study, the low activity group had fewer squamous cell cancers than the other activity groups and squamous cell cancer is the type most closely associated with smoking. It would be unlikely for this to occur if the observed association was due to non-reported smoking, rather than activity or fitness.
It is important to note that some studies have failed to report any association between physical activity and lung cancer (11, 43, 25, 7, 30, 33). Leitzmann et al (21) suggested that the inconsistent findings may be due to small sample sizes, variation in the magnitude of residual confounding by smoking, potential recall bias, or imprecise assessments of physical activity. In addition, population differences in the study cohorts, differences in lung cancer end points used (fatal, nonfatal, or combined fatal/nonfatal cases), duration of follow-up after the baseline exposure measurement, or some combination of all of the above factors may contribute to the inconsistency of results as well. While all of the previous studies except one(38) have been based on self-reported questionnaire measures, self-reported measures of physical activity are only modestly correlated with objective measures obtained using criterion methods (27, 2). The objectively measured CRF from the current study might be a more accurate and better exposure to consider. Although CRF has a genetic component (25% – 40%) (15), it is clear that usual physical activity is its primary determinant.
Some plausible mechanisms exist for a protective effect of exercise and fitness against lung cancer. There are numerous studies documenting improvement in overall immune function with increasing activity through increasing the number of natural killer cells (28). Exercise is associated with reduced systemic inflammation (particularly C-reactive protein) (18) which has been proposed to promote carcinogenesis in a wide spectrum of cancers, including lung (10). Physical activity may increase pulmonary ventilation and perfusion (8, 29), which accompany improved fitness (8), might decrease the interaction time of potential carcinogens in the airway and thus decrease the risk of lung cancer (40). Further, physical activity may enhance endogenous antioxidant defenses and reduce oxidative stress (24).
Strengths of the current study include its prospective design, maximal exercise testing to quantify CRF, and a hard end point of lung cancer mortality as the study outcome. We also were able to stratify the analyses by smoking status and lung function, which helped to shed light on some potential effect modifications. One weakness of our study is the lack of dietary data. However, a recent study that has adjusted for intakes of fruit, vegetables, and red meat found that these adjustments did not significantly change the conclusions (21). Another limitation of the current study is that the study population consists mainly of European-American men in the middle and upper socioeconomic strata; thus, the results may not be generalizable to other adult populations, however, it should not affect the internal validity. In terms of exposure assessment, we classified men at study enrollment, but in the present analysis we were unable to evaluate the effect of changes in physical activity or fitness over time on lung cancer mortality outcomes. It is possible that sedentary or low fit men increased their activity or fitness levels at some point in the follow-up interval. Additionally, others may have experienced decreases in these characteristics. Such misclassification of exposure would likely underestimate of the magnitude of the association observed in the present study. Finally, we had insufficient information to assess the effect of CRF on lung cancer incidence. Additional studies are warranted to confirm and expand on the associations we report herein and to better understand the relationship between fitness and lung cancer risk.
In summary, our data provide evidence that low levels of fitness may play a causal role in lung cancer mortality. This finding is consistent with earlier studies on self-reported physical activity and lung cancer. In addition, we observed a greater reduction in lung cancer risk than has been found in the physical activity studies. There are plausible mechanisms for a protective effect of fitness on lung cancer mortality. It is unlikely that uncontrolled, and residual confounding explain the observed association. If fitness does decrease the risk of lung cancer mortality as shown in our data, then there is something more than avoiding tobacco that can be done to lower risk of the leading cause of cancer death in the United States. The lowest risk among nonsmokers and the large reduction in risk in former smokers has important, encouraging public health implications. The consensus public health guideline (41) to obtain 150 min/wk of moderate-intensity physical activity such as brisk walking, jogging will move most of individuals out of the low-fitness category. It also may help smokers to quit smoking (22).
Acknowledgement
Supported by National Institutes of Health grants AG06945 and HL62508 and in part supported by an unrestricted research grant from The Coca-Cola Company. Results of the present study do not constitute endorsement by ACSM.
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Footnotes
Conflicts of interest: None declared.
Reference List
- 1.American Cancer Society. Cancer Facts and Figures 2009. Atlanta, GA: 2009. p. 4. [Google Scholar]
- 2.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14:422–428. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 3.Alfano CM, Klesges RC, Murray DM, Colditz GA, Stampfer MJ, Willett WC. Physical activity in relation to all-site and lung cancer incidence and mortality in current and former smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:2233–2241. [PubMed] [Google Scholar]
- 4.American College of Sports Medicine. ACSM's Guidelines For Exercise Testing And Prescription. 7th. 2005. pp. 291–294. [DOI] [PubMed] [Google Scholar]
- 5.Bak H, Christensen J, Thomsen BL, et al. Physical activity and risk for lung cancer in a Danish cohort. Int J Cancer. 2005;116:439–444. doi: 10.1002/ijc.21085. [DOI] [PubMed] [Google Scholar]
- 6.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 7.Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: further evidence from the Whitehall study. Eur J Epidemiol. 2001;17:863–869. doi: 10.1023/a:1015609909969. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YJ, Macera CA, Addy CL, Sy FS, Wieland D, Blair SN. Effects of physical activity on exercise tests and respiratory function. Br J Sports Med. 2003;37:521–528. doi: 10.1136/bjsm.37.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colbert LH, Hartman TJ, Tangrea JA, et al. Physical activity and lung cancer risk in male smokers. Int J Cancer. 2002;98:770–773. doi: 10.1002/ijc.10156. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey SG, Shipley MJ, Batty GD, Morris JN, Marmot M. Physical activity and cause-specific mortality in the Whitehall study. Public Health. 2000;114:308–315. doi: 10.1038/sj.ph.1900675. [DOI] [PubMed] [Google Scholar]
- 12.Garfinkel L, Stellman SD. Mortality by relative weight and exercise. Cancer. 1988;62:1844–1850. doi: 10.1002/1097-0142(19881015)62:1+<1844::aid-cncr2820621328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 14.Harris SR. Physical activity and breast cancer mortality. Eur J Oncol. Nurs. 2009;13:233–234. doi: 10.1016/j.ejon.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Ingelsson E, Larson MG, Vasan RS, et al. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115:2917–2924. doi: 10.1161/CIRCULATIONAHA.106.683821. [DOI] [PubMed] [Google Scholar]
- 16.Kabat GC. Aspects of the epidemiology of lung cancer in smokers and nonsmokers in the United States. Lung Cancer. 1996;15:1–20. doi: 10.1016/0169-5002(95)00566-8. [DOI] [PubMed] [Google Scholar]
- 17.Kampert JB, Blair SN, Barlow CE, Kohl HW., III Physical activity, physical fitness, and all-cause and cancer mortality: A prospective study of men and women. Ann Epidemiol. 1996;6:452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 18.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 19.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I-M, Sesso HD, Paffenbarger RSJ. Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28:620–625. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 21.Leitzmann MF, Koebnick C, Abnet CC, et al. Prospective study of physical activity and lung cancer by histologic type in current, former, and never smokers. Am J Epidemiol. 2009;169:542–553. doi: 10.1093/aje/kwn371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006219.pub2. CD006219. [DOI] [PubMed] [Google Scholar]
- 23.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 24.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:227–236. [PubMed] [Google Scholar]
- 25.Schnohr P, Gronbaek M, Petersen L, Hein HO, Sorensen TI. Physical activity in leisure-time and risk of cancer: 14-year follow-up of 28,000 Danish men and women. Scand J Public Health. 2005;33:244–249. doi: 10.1080/14034940510005752. [DOI] [PubMed] [Google Scholar]
- 26.Severson RK, Nomura AMY, Grove JS, Stemmermann GN. A prospective analysis of physical activity and cancer. Am J Epidemiol. 1989;130:522–529. doi: 10.1093/oxfordjournals.aje.a115366. [DOI] [PubMed] [Google Scholar]
- 27.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shephard RJ, Shek PN. Associations between physical activity and susceptibility to cancer: possible mechanisms. Sports Med. 1998;26:293–315. doi: 10.2165/00007256-199826050-00002. [DOI] [PubMed] [Google Scholar]
- 29.Sin DD, Jones RL, Mannino DM, Paul Man SF. Forced expiratory volume in 1 second and physical activity in the general population. Am J Med. 2004;117:270–273. doi: 10.1016/j.amjmed.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Soll-Johanning H, Bach E. Occupational exposure to air pollution and cancer risk among Danish urban mail carriers. Int Arch Occup Environ Health. 2004;77:351–356. doi: 10.1007/s00420-004-0510-9. [DOI] [PubMed] [Google Scholar]
- 31.Spence RR, Heesch KC, Brown WJ. A systematic review of the association between physical activity and colorectal cancer risk. Scand J Med Sci Sports. 2009 doi: 10.1111/j.1600-0838.2009.00992.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Sprague BL, Trentham-Dietz A, Klein BE, et al. Physical activity, white blood cell count, and lung cancer risk in a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:2714–2722. doi: 10.1158/1055-9965.EPI-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4:807–811. [PubMed] [Google Scholar]
- 34.Steindorf K, Friedenreich C, Linseisen J, et al. Physical activity and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Int J Cancer. 2006;119:2389–2397. doi: 10.1002/ijc.22125. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 36.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–615. doi: 10.1016/j.amjhyper.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tardon A, Lee WJ, gado-Rodriguez M, et al. Leisure-time physical activity and lung cancer: a meta-analysis. Cancer Causes Control. 2005;16:389–397. doi: 10.1007/s10552-004-5026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31:764–769. doi: 10.2337/dc07-1648. [DOI] [PubMed] [Google Scholar]
- 39.Thune I, Lund E. The influence of physical activity on lung-cancer risk: A prospective study of 81,516 men and women. Int J Cancer. 1997;70:57–62. doi: 10.1002/(sici)1097-0215(19970106)70:1<57::aid-ijc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 41.U.S Department of Health and Human Services. [web access date 10/7/2008];2008 Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/guidelines/default.aspx.
- 42.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85:1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]