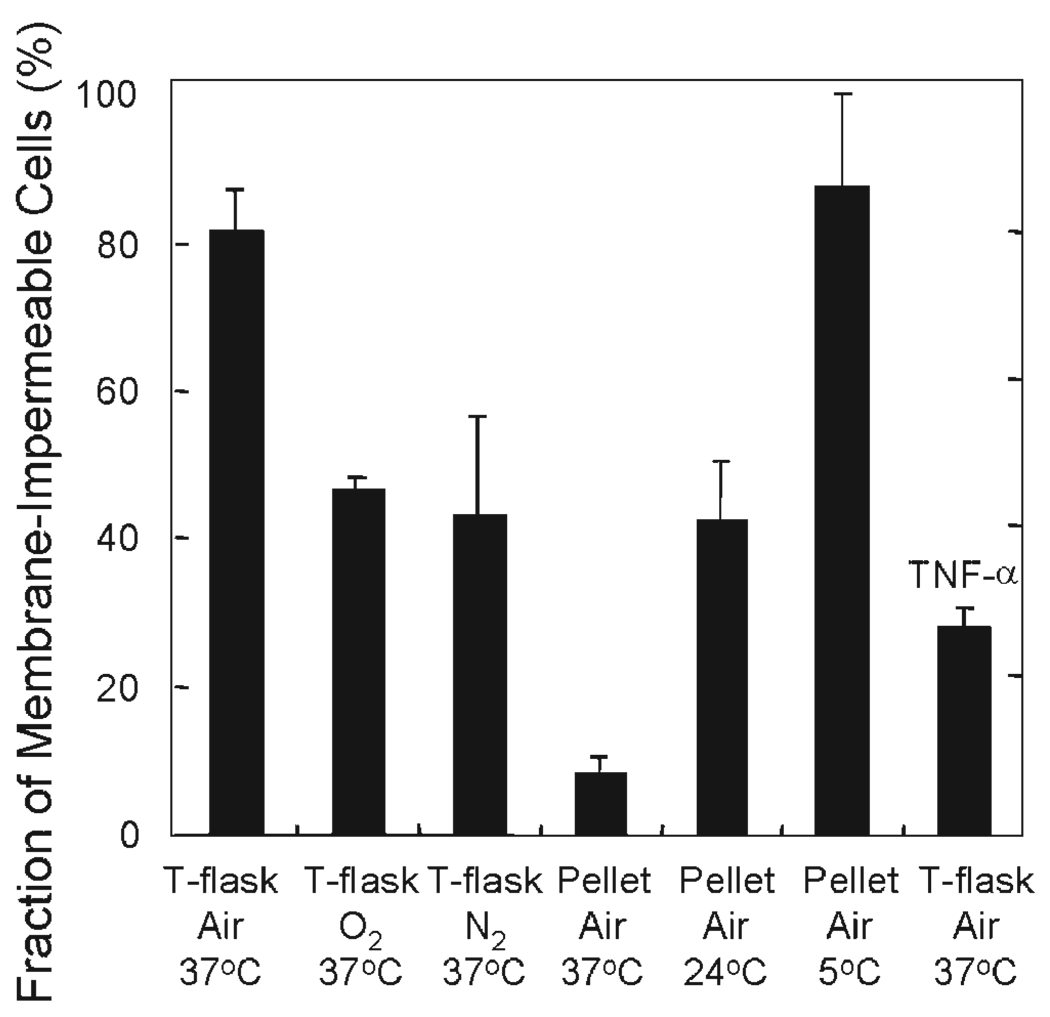
Figure 5.
Fraction of βTC3 cells that were membrane-impermeable after culture under normal and a variety of stressful conditions for 24 h. Approximately 2 × 107 βTC3 cells were used in each experiment. For surface-attached cultures, cells were cultured in T-75 flasks in a 37°C incubator at a plating density such that they would not be confluent after 24 h. Medium depth was 2 mm. For pelletized culture, cells were placed in 15 mL centrifuge tubes with 15 mL culture media and let settle by gravity, leading to cell depth of about 3 mm overlain by 11 cm of media. The tubes were sealed and cultured at temperatures of 5, 24, and 37°C. For hyperoxic and anoxic culture, flasks, and tubes were placed in sealed chambers within an incubator, and the sealed chambers were continuously flushed with premixed gases (95% O2 or 95% N2, balance CO2). At the end of the 24-h culture, cells in flasks were trypsinized. All cells from each condition were collected and samples for OCR and trypan blue staining were taken. Fraction of membrane-impermeable cells was determined as the number of cells that did not take up trypan blue divided by the total number of cells counted.